Fig. 12.1
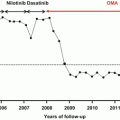
Rates of treatment-free remission in the (a) STIM and (b) TWISTER clinical trials. Kaplan-Meier estimates of the proportion of patients free of molecular recurrence after imatinib withdrawal. The solid line indicates the Kaplan-Meier estimate of the probability of remaining in TFR. Dashed lines (TWISTER) indicate the 95 % confidence interval. Relapse was defined as loss of UMRD confirmed on a second sample (TWISTER) or followed by a onefold rise in BCR-ABL (STIM) (Mahon et al., ASH 2013 [abstract 255]).
STIM, A-STIM, and TWISTER enrolled a mixture of patients treated second line with imatinib after interferon and those treated first line. All studies showed a higher rate of TFR in the patients previously treated with IFN (around 50 %) versus first-line patients (around 30 %) [23–25]. This analysis is confounded by the fact that patients who remained on IFN for long enough to switch to imatinib may have had more favourable disease biology to begin with. The heterogeneous patient population also confounds any attempt to identify characteristics that predict successful TFR (see Sect. 12.4). The STIM2 study, which is enrolling only first-line imatinib-treated patients, may help to clarify some of these questions. An interim analysis of STIM2 reported 61 % of patients still in MMR at a median of 12 months after imatinib discontinuation [26]. Interestingly, more than half of the patients in MMR had detectable BCR-ABL transcripts at a low level, and 28 % of patients maintained UMRD, consistent with the earlier studies. Why this persistent disease does not inevitably lead to relapse remains a key unanswered question.
12.3.4 Newer TKIs
Prospective multicentre studies of TFR after response to nilotinib and dasatinib are currently underway, but the published experience of withdrawal of second-generation TKIs is currently limited to registry data and small series. Réa and colleagues reported a series of 34 patients with at least 6 months of follow-up, and the probability of stable MMR after 12 months off TKI was 58 % [27]. The limited results available indicate a TFR rate at least equivalent to that seen after imatinib withdrawal. Since deeper molecular responses are seen earlier and more frequently in patients treated with more potent TKIs [3, 28], there is an expectation that the increasing use of second-generation TKIs may result in a larger proportion of patients being eligible for a trial of TFR.
12.3.5 Higher-Risk Patients
The STIM study showed a strong association of Sokal score with molecular relapse [23]. It is remarkable that simple clinical markers of risk at diagnosis should still have an impact on the probability of TFR many years later, in spite of deep molecular response. This suggests that previous disease biology remains relevant and cautions us that the hazards of TKI discontinuation may be greater in higher-risk patients. A French study of stopping dasatinib or nilotinib in UMRD reported that the rate of TFR among patients on second-line therapy for poor response was around half of that seen in patients treated first line with dasatinib/nilotinib or second line after imatinib intolerance [27]. Nevertheless, there are rare reports of TFR among patients achieving a deep molecular response following treatment for imatinib-resistant disease with kinase domain mutations or even after blast crisis [29, 30]. It should be noted that most of these patients stopped the TKI due to toxicity concerns. Patients with kinase domain mutations or advanced phase CML are generally excluded from TFR studies, and elective TKI discontinuation is not recommended in this population.
12.4 Relapse Definitions
The earliest TFR studies used conservative criteria for restarting TKI therapy, since the safety of drug withdrawal was not known. As the volume of clinical experience has increased, it has become apparent that molecular relapse can be rescued by the reintroduction of the original TKI, that TKI resistance at relapse is not a problem, and that low-level molecular positivity does not inevitably lead to a progressive rise in disease burden. Criteria for restarting TKI in selected TFR studies are shown in Table 12.1. STIM and TWISTER mandated restarting treatment if BCR-ABL mRNA was detectable in two consecutive samples. Some patients were seen in whom intermittent positive samples did not lead to loss of MMR [24]. This observation was examined in A-STIM, where the trigger to restart TKI was loss of MMR. The kinetics of low-level BCR-ABL revealed that around half of patients who lose UMRD will remain in MMR without treatment [25]. Importantly, MMR was maintained with longer follow-up, with very few relapses occurring later than 2 years.
Table 12.1
Summary of key features of TFR studies reported to date
Relapse criterion | ||||
|---|---|---|---|---|
Treatment | Loss of UMRD (TWISTER [24]) | Loss of UMRD and tenfold rise in BCR-ABL (STIM [23]) | Loss of MMR (A-STIM [25]) | |
STIM2 [26] | First-line imatinib | X | ||
HOVON51 [31] | Imatinib + cytarabine | X | ||
DADI [32] | Dasatinib | X | ||
ENESTfreedoma | Nilotinib | X | ||
DASfreea | Dasatinib | X |
12.5 Eligibility for a Trial of TFR
Just as the relapse definitions were conservative in the earliest studies, so too were the inclusion criteria. No detectable BCR-ABL in any sample in the 2 preceding years was required in STIM and TWISTER. Since loss of UMRD does not translate to clinical relapse, it is logical to ask whether less stringent molecular responses can be accepted prior to attempting TFR. A further, and important, motivation to change the inclusion criteria for TFR comes from the difficulty of standardizing UMRD (discussed in Sect. 12.2). Ongoing studies are using sustained MR4.5 or MR4 as qualifying levels of molecular response for a trial of TFR.
The A-STIM trial allowed the enrolment of patients with a less stringent definition of UMRD that allowed occasional low-level positive results within the preceding 2 years. The rate of treatment-free MMR in those patients was identical to patients meeting the stringent UMRD criterion, but the rate of treatment-free UMRD was significantly lower [25]. It remains to be seen whether or not this difference is clinically relevant with longer follow-up.
12.6 Biology of TFR
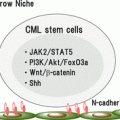
Whereas the achievement and maintenance of deep molecular response appears to be a prerequisite for TFR, a low level of minimal residual disease is not sufficient to guarantee that a patient will sustain TFR. This means that TFR should be thought of as a distinct biological state, the determinants of which remain to be elucidated. There are at least three possible elements that define TFR: the first is the amount of residual CML; the second is the quality of the residual CML clone; and the third comprises factors extrinsic to the CML clone, such as stromal interactions or immunological surveillance [33].
The first studies of TFR included patients with UMRD (based on conventional RQ-PCR) with the simple idea that in some cases the CML clone was completely eradicated and that if only we could identify these patients, we could select those who no longer needed TKI treatment. In fact, it is now clear that most, or even all, patients who remain in TFR still have residual CML cells. A limitation of conventional RQ-PCR for BCR-ABL for very low levels of MRD is the risk of false-positive results that may occur due to cross-contamination between samples processed in the same laboratory or due to rare BCR-ABL transcripts that can be found in normal individuals [34]. An assay for patient-specific BCR-ABL intronic DNA sequences virtually eliminates the risk of false-positive results because each patient’s breakpoint is essentially unique, whereas the vast majority of CML patients have one or both of the two common mRNA transcripts [35–37]. Using a semi-quantitative DNA PCR approach, it was shown that all 26 patients tested in the TWISTER study had detectable CML cells on at least one occasion during follow-up. There was no significant difference in the risk of relapse when comparing those with and without detectable BCR-ABL DNA prior to imatinib withdrawal, but the number of patients assessed was too small to draw definitive conclusions on the relationship between depth of response and relapse risk [24]. The nature of the cells that are providing the positive DNA signal was not studied, but in A-STIM, an analysis of three patients with fluctuating low-level BCR-ABL showed that BCR-ABL mRNA transcripts were enriched in the CD15+ myeloid fraction, and not in B cells or T cells [25]. It is possible that there is an MRD threshold (somewhere around MR4.0) above which TFR cannot be achieved. What is not yet clear is whether deeper levels of response (e.g. MR5.0 to MR6.0) are associated with a lower risk of molecular recurrence, since such levels of response are not accurately quantifiable with routine assays.
The association of Sokal score with the risk of molecular recurrence many years later argues that factors intrinsic to the leukemic clone still play an important role in TFR biology. Multiple biological parameters (e.g. OCT-1 activity [38], KIR genotype [39]) have been reported to influence the probability of achieving molecular response, but none has yet emerged as a reliable predictor of TFR after deep molecular response has been achieved. A major obstacle to studying this question is the time elapsed between diagnosis and a trial of TFR: this means that relatively few patients will have available diagnostic material for study.
Immunological function is the best-studied of the CML-extrinsic factors that may be relevant to TFR. It was already mentioned above that cytotoxic T lymphocytes reactive against myeloid antigens are associated with stable or deepening molecular response after TKI withdrawal in patients who continue IFN [22]. NK cell numbers are the only immunological parameter to emerge as a potential predictor of TFR in clinical studies. A Japanese study identified increased CD3-CD56+ NK cell numbers in patients in TFR after imatinib withdrawal, but NK cell numbers were not measured prior to TKI withdrawal so the predictive value of NK cell numbers could not be assessed [40]. IMMUNOSTIM, a scientific substudy of the original STIM trial reported that mean NK cell numbers at study entry were higher in patients who maintained TFR than in those who relapsed [41]. The difference was most striking in the CD56dim population of NK cells that is thought to have the greatest cytotoxic activity. The range of NK cell numbers in the TFR group and the relapse group overlapped considerably. At present, there is no robust immunological predictor of TFR.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree