In some clinical settings laboratory measurement of direct oral anticoagulants effect is helpful in guiding medical care, such as life-threatening bleeding, need for emergency surgery, renal impairment, severe hepatic failure, extremes of body weight, or in patients with bleeding or thrombosis on therapy. This article reviews approaches to laboratory testing to assess the anticoagulant effect of these drugs. Because of the wide variation in levels measured in patients on therapy and minimal clinical data from dose adjustment, dose adjustment based on levels is not currently advised. In addition, these drugs interfere with many clot-based laboratory tests and caution is advised in interpreting these tests in patients on direct oral anticoagulants.
Key points
- •
The direct oral anticoagulants (DOACs), dabigatran, apixaban, edoxaban, and rivaroxaban, were approved for prevention and treatment of venous thrombosis and for prevention of embolic stroke in patients with atrial fibrillation, without need to monitor drug activity levels.
- •
Clinical circumstances exist where laboratory measures of drug activity may help guide clinical care.
- •
Trough drug levels correlate best with bleeding risk, although values measured in patients on the medications vary widely.
- •
Screening laboratory tests differ in their sensitivity to the drugs and knowledge of the assays available and laboratory-specific performance is required.
- •
Direct oral anticoagulants can affect clot-based coagulation assays performed, including tests for thrombophilias, factor levels, and thromboelastography, and results of those tests should be interpreted with caution in patients on DOACs.
Introduction
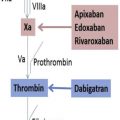
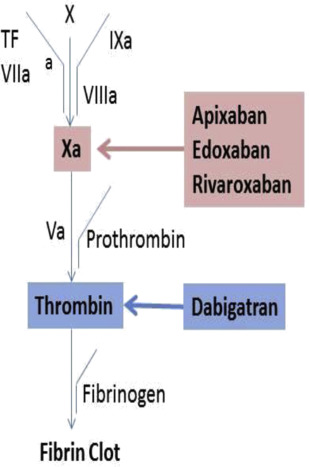
Direct oral anticoagulants (DOACs) inhibit coagulation through factor Xa (apixaban, edoxaban, rivaroxaban) or thrombin (dabigatran) ( Fig. 1 ), and do not require antithrombin for activity as is needed for the heparins and fondaparinux. DOACs are approved for prevention and treatment of venous thromboembolism and for treatment of patients with atrial fibrillation. Clinical trials were designed and regulatory approval given without a need for dose adjustment based on laboratory testing. In clinical trials use of these agents was associated with less or similar bleeding and thrombotic complications compared with warfarin. However, in certain clinical settings measurement of anticoagulant activity is desired to help inform patient care. These include life-threatening bleeding, emergency surgery, renal impairment, liver failure, in patients taking medications that affect DOAC plasma concentrations, recurrent thrombosis or bleeding on recommended doses, or extremes of body weight ( Box 1 ).
Major bleeding
Emergent need for surgery
Renal impairment
Severe liver failure
Potential drug-drug interactions
Bleeding or thrombosis on therapy
Extremes of body weight
True therapeutic ranges based on clinical outcomes have not been established for these agents. Levels that correlate with efficacy and/or adverse outcomes are just now being studied. Instead we have levels that were measured in study populations on standard dosing, most commonly at peak and trough concentrations using liquid chromatography/tandem mass spectrometry (LC-MS/MS) methodology. Of note, these levels vary widely across study participants. Instead of therapeutic levels, the terms on-therapy drug concentrations or on-therapy levels are more accurate.
The pharmacology of the DOACs is discussed in detail elsewhere (see Robert I. Handin’s article, “ The History of Antithrombotic Therapy — The Discovery of Heparin, the Vitamin K Antagonists and the Utility of Aspirin ,” in this issue). Overall the drugs are similar in peak levels (1–4 hours) and half-life (approximately 7–12 hours). The latter depends, in part, on age and comorbid conditions. Additional key drug characteristics when considering laboratory monitoring are shown in Table 1 .
| Drug | Drug Targets (Direct Inhibitors) | % Renal Clearance | Measured Peak Level (ng/mL) | Measured Trough Level (ng/mL) |
|---|---|---|---|---|
| Dabigatran | Thrombin | 80 | 175 (CV, 74%) a | 91 (CV, 82%) a |
| Rivaroxaban | Factor Xa | 36 | 215 (22–535) b | 32 (6–239) b |
| Apixaban | Factor Xa | 25 | 129 (CV, 10%) c | 50 (20%) c |
| Edoxaban | Factor Xa | 50 | n/a | 36 (IQR, 19–62) d |
a Mean concentration and CV in patients with atrial fibrillation on 150 mg twice daily.
b Steady state mean in healthy volunteers given 10 mg daily with range given in parenthesis.
c Steady state mean concentration in six healthy volunteers given 5 mg twice daily with CV given.
d Median value in patients with atrial fibrillation given 60 mg daily with IQR given.
Introduction
Direct oral anticoagulants (DOACs) inhibit coagulation through factor Xa (apixaban, edoxaban, rivaroxaban) or thrombin (dabigatran) ( Fig. 1 ), and do not require antithrombin for activity as is needed for the heparins and fondaparinux. DOACs are approved for prevention and treatment of venous thromboembolism and for treatment of patients with atrial fibrillation. Clinical trials were designed and regulatory approval given without a need for dose adjustment based on laboratory testing. In clinical trials use of these agents was associated with less or similar bleeding and thrombotic complications compared with warfarin. However, in certain clinical settings measurement of anticoagulant activity is desired to help inform patient care. These include life-threatening bleeding, emergency surgery, renal impairment, liver failure, in patients taking medications that affect DOAC plasma concentrations, recurrent thrombosis or bleeding on recommended doses, or extremes of body weight ( Box 1 ).
Major bleeding
Emergent need for surgery
Renal impairment
Severe liver failure
Potential drug-drug interactions
Bleeding or thrombosis on therapy
Extremes of body weight
True therapeutic ranges based on clinical outcomes have not been established for these agents. Levels that correlate with efficacy and/or adverse outcomes are just now being studied. Instead we have levels that were measured in study populations on standard dosing, most commonly at peak and trough concentrations using liquid chromatography/tandem mass spectrometry (LC-MS/MS) methodology. Of note, these levels vary widely across study participants. Instead of therapeutic levels, the terms on-therapy drug concentrations or on-therapy levels are more accurate.
The pharmacology of the DOACs is discussed in detail elsewhere (see Robert I. Handin’s article, “ The History of Antithrombotic Therapy — The Discovery of Heparin, the Vitamin K Antagonists and the Utility of Aspirin ,” in this issue). Overall the drugs are similar in peak levels (1–4 hours) and half-life (approximately 7–12 hours). The latter depends, in part, on age and comorbid conditions. Additional key drug characteristics when considering laboratory monitoring are shown in Table 1 .
| Drug | Drug Targets (Direct Inhibitors) | % Renal Clearance | Measured Peak Level (ng/mL) | Measured Trough Level (ng/mL) |
|---|---|---|---|---|
| Dabigatran | Thrombin | 80 | 175 (CV, 74%) a | 91 (CV, 82%) a |
| Rivaroxaban | Factor Xa | 36 | 215 (22–535) b | 32 (6–239) b |
| Apixaban | Factor Xa | 25 | 129 (CV, 10%) c | 50 (20%) c |
| Edoxaban | Factor Xa | 50 | n/a | 36 (IQR, 19–62) d |
a Mean concentration and CV in patients with atrial fibrillation on 150 mg twice daily.
b Steady state mean in healthy volunteers given 10 mg daily with range given in parenthesis.
c Steady state mean concentration in six healthy volunteers given 5 mg twice daily with CV given.
d Median value in patients with atrial fibrillation given 60 mg daily with IQR given.
Diagnostic options
Direct Oral Anticoagulants and Coagulation Testing
Although clinicians may consider measurement of DOAC-induced anticoagulant activity in several settings, they should be cautioned before testing clinically stable patients, given the wide range of on-therapy drug concentrations that have been measured in subjects receiving standard dosing. Also, given the relatively short half-life of these drugs, the levels are not a measure of drug adherence as the international normalized ratio (INR) is for warfarin therapy. If activity is measured it must be timed to assess trough or peak activity, so that results are interpreted in light of published data. Because there is more variability in peak concentrations among the DOACs, trough levels are recommended if steady-state levels are being assessed. To date, no laboratory assays developed specifically to measure DOAC activity have been approved by the Food and Drug Administration. There is no evidence to support dose adjustment based on test results.
Screening Coagulation Tests (Prothrombin Time and Activated Partial Thromboplastin Time) and Direct Oral Anticoagulants
The prothrombin time (PT) measures levels of the coagulation proteins in the classical extrinsic and common pathways ( Fig. 2 ). For this assay platelet-poor plasma is prepared from sodium citrate–anticoagulated whole blood. Coagulation is activated in vitro by the addition of calcium and a thromboplastin reagent, which contains a source of tissue factor and phospholipid. The laboratory result is the time to clot formation.
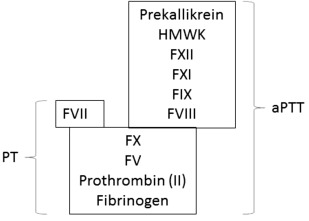
Because there is variability in sensitivity to factor deficiencies depending on the source of tissue factor and phospholipid used for the PT assay, the INR was introduced to standardize results. The INR equals (patient PT/mean normal PT) ISI . The international sensitivity index (ISI) reflects the responsiveness of each thromboplastin reagent to reductions in the vitamin K–dependent clotting factors. Recombinant tissue factor is assigned an ISI of 1.0. It is important to remember that the ISI reflects the sensitivity to the effect of warfarin on the PT and may not reflect factor activities influenced by other drugs or medical conditions. The calculation and use of the INR using the ISI established for monitoring of warfarin anticoagulation is not valid for rivaroxaban, or other Xa inhibitors, because this has been shown to increase the drug-induced between-thromboplastin variability.
The activated partial thromboplastin time (aPTT) measures levels of the coagulation proteins in the classical intrinsic and common pathways (see Fig. 2 ). The term “partial thromboplastin” was first used to describe the reagent because activation of clotting in the test did not correct the deficit in hemophilic plasma. This is in contrast to the “complete thromboplastin” phospholipid and tissue factor used in the PT. In the 1960s, the addition of a surface activator (eg, silica, ellagic acid, or kaolin) became standard because it improves assay precision and sensitivity to factor deficiencies. As for the PT, platelet-poor plasma prepared from sodium citrate–anticoagulated whole blood is used. Coagulation is activated in vitro by the addition of calcium and a surface activator. The laboratory result is the time to clot formation.
DOACs differ in their effect on the PT and aPTT and neither of these assays can be used to exclude significant drug concentrations of dabigatran, rivaroxaban, or apixaban unless laboratory-specific assay sensitivity to the drug is known ( Table 2 ).
| Drug a | Clinical Scenario | ||
|---|---|---|---|
| Exclude Relevant Anticoagulant Effect | Detect Overanticoagulation | Monitor Drug Activity | |
| Dabigatran | TCT | aPTT, TCT, ECA, ECT | Dilute TCT c , ECA, ECT |
| Rivaroxaban | Anti-Xa a | PT, anti-Xa b | PT d , anti-Xa b |
| Apixaban | Anti-Xa a | Anti-Xa b , PT only if sensitivity established in local laboratory | Anti-Xa b |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree