In a 52-week active-comparator study, patients not achieving adequate glycaemic control on a metformin dose of at least 1500 mg/day were randomized to sitagliptin 100 mg once daily or glipizide, which was initiated at a dose of 5 mg/day. Up-titration of glipizide was performed over 18 weeks to a maximum dose of 20 mg, after which no increase in glipizide dose was permitted. Sitagliptin added to ongoing metformin therapy demonstrated an identical 0.67% HbA1c reduction to glipizide plus metformin therapy in a patient population with predominantly mild to moderate hyperglycaemia (Nauck et al., 2007). In this study, 187 (32.0%) glipizide-treated patients reported 657 episodes of hypoglycaemia compared with 29 (4.9%) sitagliptin-treated patients who reported 50 episodes of hypoglycaemia. At 52 weeks, body weight was significantly reduced with sitagliptin (-1.5 kg) and significantly increased with glipizide (+1.1 kg) relative to baseline (Figure 5.1).
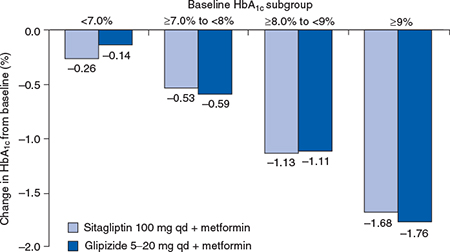
In all the pivotal sitagliptin studies, greater reductions in HbA1c were achieved in patients with higher HbA1c levels at baseline (Figure 5.2). Sitagliptin therapy reduced both fasting and postprandial plasma glucose, in association with improvements in the proinsulin:insulin ratio and homeostatic model assessment of beta-cell function (HOMA-B).
Vildagliptin
In an extensive clinical trial programme, vildagliptin at 50 mg once or twice daily and 100 mg once daily has been assessed in a number of placebo-controlled and active-comparator monotherapy studies (Dejager et al., 2007; Pi-Sunyer et al., 2007; Rosenstock et al., 2007a; Schweizer et al., 2007). Pooled data from these monotherapy trials in 1469 drug-naїve patients showed that vildagliptin 100 mg daily produced an adjusted mean change in HbA1c of -1.0% at 24 weeks from a mean baseline value of 8.6% (Rosenstock and Fitchet, 2008). Two active-controlled monotherapy studies have compared vildagliptin 50 mg twice daily with either metformin 1000 mg twice daily (Schweizer et al., 2007) or rosiglitazone 8 mg once daily (Rosenstock et al., 2007a). Vildagliptin did not demonstrate non-inferiority to metformin in the one-year trial, although significant reductions in HbA1c from baseline were achieved (Schweizer et al., 2007). In contrast, in a 24-week study, vildagliptin was as effective as rosiglitazone with adjusted mean changes in HbA1c of – 1.1% versus -1.3% from a mean baseline of 8.7% (Rosenstock et al., 2007a).
Figure 5.1 Effects of sitagliptin versus glipizide on body weight in patients with type 2 diabetes. Reproduced from Nauck et al. (2007) with permission from Wiley-Blackwell.
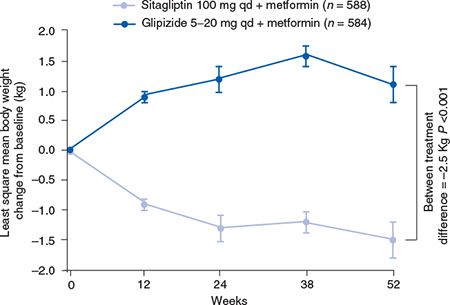
Figure 5.2 Mean HbA1c change (± SE) from baseline at Week 52 by baseline HbA1c subgroups in the sitagliptin active-comparator study with glipizide. Reproduced from Nauck et al . (2007) with permission from Wiley-Blackwell.
In a series of 24-week, 3-arm studies, vildagliptin 50 mg once daily, vildagliptin 50 mg twice daily and placebo have been compared as addon combination therapy in patients with inadequate glycaemic control on metformin (Bosi et al., 2007), a sulphonylurea (Garber et al., 2008), or a thiazolidinedione (Garber et al., 2007) (Table 5.3). In patients on metformin monotherapy, the two vildagliptin doses significantly decreased HbA1c relative to placebo by 0.7% and 1.1%, respectively, although the difference between doses was not statistically significant (Bosi et al., 2007). As add-on to glimepiride 4 mg per day, both vildagliptin doses in combination produced reductions in HbA1c of 0.6% at 24 weeks, which were statistically significant compared with the 0.1% HbA1c increase in the glimepiride plus placebo group (Garber et al., 2008). Added to a maximum dose of pioglitazone, vildagliptin doses produced significant reductions from baseline of 0.8% and 1.0%, respectively, compared with a 0.3% reduction in patients receiving placebo plus pioglitazone (Garber et al., 2007).
In a study of vildagliptin 50 mg twice daily or placebo as add-on to insulin, HbA1c was reduced by 0.5% with vildagliptin at 24 weeks compared with 0.2% for placebo (Fonseca et al., 2007). The improvements in HbA1c with vildagliptin occurred in association with a lower total daily insulin dose and no episodes of significant hypoglycaemia compared with six episodes in those treated with insulin plus placebo.
A 24-week study has evaluated vildagliptin and pioglitazone as dual therapy compared with either agent as monotherapy in drug-naїve patients with type 2 diabetes (Rosenstock et al., 2007b). From baseline to 24 weeks the vildagliptin 100 mg/day monotherapy group demonstrated a statistically significant reduction in HbA1c of 1.1% compared with 1.4% for pioglitazone 30 mg/day monotherapy. Dual therapy demonstrated a 1.9% reduction in HbA1c for the high dose dual therapy group (vildagliptin 100 mg plus pioglitazone 30 mg daily) and 1.7% in the low dose dual therapy group (vildagliptin 50 mg plus pioglitazone 15 mg daily).
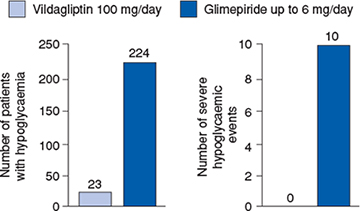
Two active-comparator trials in patients with inadequate glycaemic control while receiving a stable metformin dose (at least 1500 mg/day) have evaluated vildagliptin compared with pioglitazone (Bolli et al., 2008) and compared with glimepiride (Ferrannini et al., 2009). In the 24-week pioglitazone active comparator study, non-inferiority of vildagliptin to pioglitazone as addon to metformin was established (Bolli et al., 2008). In a large two-year study, a pre-planned interim analysis at one year showed a mean change in HbA1c from baseline to endpoint of -0.4% in vildagliptin plus metformin-treated patients and -0.5% in glimepiride plus metformin-treated patients, establishing non-inferiority of vildagliptin to glimepiride as add-on to metformin (Ferrannini et al., 2009). No weight gain occurred in vildagliptin-treated patients in contrast to those receiving glimepiride, resulting in a significant between-group difference of 1.79 kg at the end of the study. Vildagliptin was also associated with a tenfold lower incidence of hypoglycaemia than glimepiride: 23 (1.7%) versus 224 (16.2%) of patients presenting with at least one hypoglycaemic event, with no reports of severe events compared with 10 severe hypoglycaemic events in the glimepiride group (Figure 5.3).
Table 5.3 HbA1c-lowering efficacy of vildagliptin in combination with other antidiabetes agents
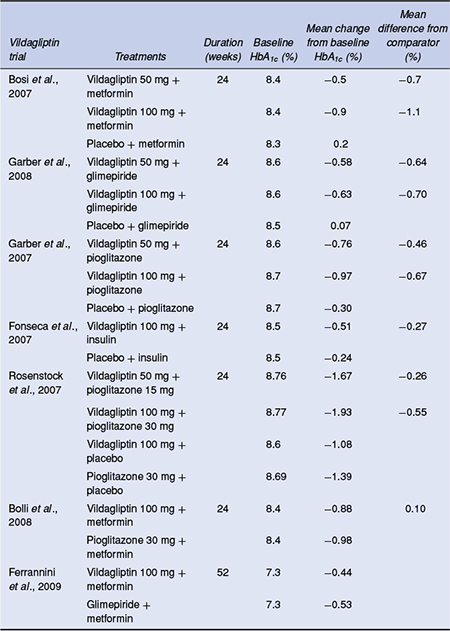
Figure 5.3 Incidence and severity of hypoglycaemic events with vildagliptin and glimepiride during the 52-week treatment period. Reproduced from Ferrannini et al. (2009) with permission from Wiley-Blackwell.
Vildagliptin-treated patients did not demonstrate weight gain in any of the above studies and the risk of hypoglycaemia for vildagliptin as add-on to metformin was very low and comparable with placebo and no episodes of severe hypoglycaemia were recorded.
Saxagliptin
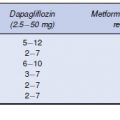
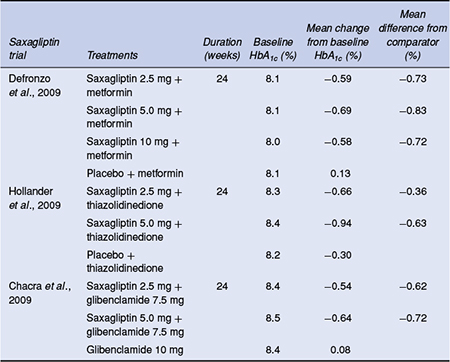
Six pivotal Phase 3 trials have reported on the efficacy of saxagliptin as monotherapy, as well as in combination with metformin, sulphonylureas and thiazolidinediones at saxagliptin doses of 2.5,5, and 10 mg (Table 5.4).
The efficacy of saxagliptin as monotherapy was studied in two 24-week, double-blind, placebo-controlled trials. In treatment-naїve patients, once-daily saxagliptin monotherapy for 24 weeks demonstrated clinically significant reductions in HbA1c, fasting and postprandial plasma glucose at all doses compared with placebo (Rosenstock et al., 2009). In a second monotherapy study the efficacy of initial combination therapy with saxagliptin and metformin was compared with saxagliptin and metformin monotherapy in treatment-naїve type 2 diabetes patients with inadequate glycaemic control (Jadzinsky et al., 2009). Saxagliptin in combination with metformin as initial therapy led to statistically significant improvements compared with either treatment alone across all glycaemic parameters with a tolerability profile similar to the monotherapy components.
Table 5.4 HbA1c-lowering efficacy of saxagliptin in combination with other antidiabetes agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree