Fig. 9.1
GASC features. The scheme depicts the protocol used to isolate GASC, the GASC features that have been analyzed and the assays used
9.3.1 GASC Are a Population of Stem Cells with Tumor-Supporting Function
Since 2006, the above-mentioned protocol has been applied to both high-grade and low-grade gliomas of the supratentorial region. Despite the stringent culture conditions, proliferating cell lines were obtained from about 95% of both LGG and high-grade gliomas (HGG). We named these cells Glioma-Associated Stem Cells or GASC. To date, our cell bank includes more than 250 GASC lines, indicative of the high efficiency of the optimized method.
To demonstrate whether GASC possessed stem cell properties we investigated whether in vitro they display an undifferentiated phenotype as well as clonogenicity and multipotency, two stemness-related properties (Fig. 9.1).
Indeed, since one week from seeding, proliferating cells, independently from the grade of the glioma of origin, displayed a fibroblast-like morphology and were highly positive for the expression of intermediate filaments characterizing an undifferentiated state (e.g. vimentin and nestin), while the glial fibrillary acidic protein (GFAP) was expressed only in a minority of the cells. GASC also expressed the pluripotent-state specific transcription factors Oct-4, Nanog and Sox-2. All these features were retained at the third passage in culture. Regarding their surface phenotype, GASC presented a mesenchymal phenotype as assessed by flow-cytometry testing a wide panel of positive/negative markers, according to the International Society for Cellular Therapy [56].
In order to test whether GASC were characterized by stem cell properties, a single-cell cloning assay was performed demonstrating the presence of self-renewing clonal cells that maintained a stable undifferentiated phenotype.
Moreover, when cultured under appropriate differentiation-inducing conditions, clonal GASC acquired lineage-specific features. Specifically, when exposed to a neural differentiation medium, cells displayed morphological changes and differentiated into neuronal-like, glial-like and oligodendrocyte-like cells. Similarly to MASC isolated from normal tissues [44–53], GASC retain also the ability to differentiate along mesodermic (e.g. endothelial-, osteoblast- and myocyte-like cells), and endodermic (e.g. hepatocyte-like cells) derivatives, although with a very low efficiency.
However, despite some similarities, the growth properties of GASC were significantly different from those of MASC obtained from normal tissues [44–53]. Specifically, GASC displayed an anchorage-independent growth, being able to grow as spheroids when seeded into a soft agar. This aberrant growth property is typical of tumor cells but can be also presented by tumor-supporting cells.
To exclude the possible neoplastic nature of GASC, a whole genome Single Nucleotide Polymorphism (SNP) analysis was performed. Indeed comparing the SNP profile of GASC-lines with the ones of the respective tumor of origin, all tested GASC were devoid of the genetic alterations characterizing the matched glioma tissues. Accordingly, when injected into the striatum of NOD-Scid mice, 105 either polyclonal or clonal GASC were unable to give rise, even after 8 months, to tumors, excluding their tumor-initiating capacity.
To test instead the tumor-supporting ability of GASC, two commercially available glioblastoma cells lines (A172 and U87) were grown in the presence of the supernatant of GASC. Both cell lines, when conditioned by GASC, displayed a significantly increased growth kinetic and capacity to grow in soft agar. Interestingly the tumor-supporting ability was increased in GASC isolated from high-grade glioma, with respect to those obtained from LGG.
More recently, unpublished data from our laboratory shows that GASC possess a tumor-supporting function also when injected in vivo into an orthotopic murine model of glioblastoma.
In conclusions, both LGG and HGG contain a population of cells characterized by stem cell properties and aberrant growth properties. These cells are not tumor-initiating cells but act as tumor-supporting cells.
9.3.2 How GASC Exert Their Tumor-Supporting Function
Understanding the mechanism through which GASC support the tumor growth can open the way to novel therapeutic strategies aimed at interfering with this harmful cross talk. Up to now, we have determined that GASC can act on tumor cells by releasing exosomes [7] and by modulating GSC adhesive properties [57].
9.3.2.1 GASC Act Through the Release of Exosomes
It has been recently shown that the potent cross talk between cancer cells and tumor-associated stromal cells can be, at least in part, mediated by the release of exosomes [58–61]. These latter are extracellular vesicles, smaller than 150 nm in diameter, that originate in the multivesicular body compartment and are released into the extracellular space and in the body fluids from many cell types [58–62]. Since exosomes can deliver to target cells their content of biologically active molecules (e.g. proteins, mRNAs, miRNAs, lncRNAs), thus modifying their physiological state, they act as a potent intercellular communication system [61, 63–65].
At this regard, Skog’s group showed that glioblastoma tumor cells could release exosomes, containing mRNA, miRNA and angiogenic proteins, able to act on endothelial cells, possibly favoring the development of a tumor-permissive microenvironment [66]. Interestingly, exosomes can be also released into the bloodstream [66] and act on distant sites taking part to the formation of a pre-metastatic niche [59, 67, 68]. Conversely, it has been shown that tumor-associated fibroblasts, through the release of exosomes, could induce, in breast tumor cells, the acquisition of a metastatic phenotype [60].
For this reason, we wondered whether GASC could exert their tumor-supporting action in this way [7]. Exosomes were therefore isolated from GASC culture supernatants and their presence was confirmed by Atomic Force Microscopy and Light Scattering (particles with a diameter ranging from 20 to 110 nm) (Fig. 9.2).
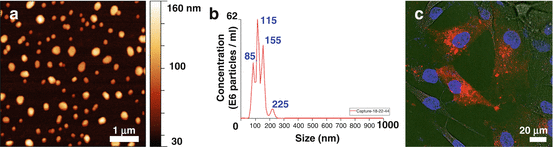

Fig. 9.2
GASC release exosomes in the supernatant. (a) Atomic force microscopy topographic height of GASC-derived exosomes. The exosomes appear as circular structures with diameters ranging from 20 to 120 nm. (b) Size distribution (diameter in nm) and concentration of exosomes resulting from the Nanoparticle Tracking Analysis. (c) DiD-labeled exosomes (red fluorescence) appear to be internalized by glioblastoma cells (overlaid phase-contrast image). Nuclei are depicted by the blue fluorescence of DAPI
In order to establish whether glioblastoma cells could incorporate GASC-derived exosomes, we labeled these latter with the lipophilic DiD-dye and, as shown in Fig. 9.2, after 4 h of incubation, DiD-labeled exosomes could be identified within the cells [7].
To unequivocally demonstrate that the tumor-supporting fraction of GASC supernatant was indeed due to exosomes and not to other soluble factors, we compared the capacity of: un-fractioned GASC supernatants, exosome-depleted GASC supernatants and GASC-derived exosomes to modify growth kinetic, migration ability and anchorage-independent growth of A172 cells, showing that indeed exosomes were the main responsible for the effects observed. Same effects were observed when we evaluated a clinically more relevant experimental setting, isolating from the same HGG, both GASC and GSC and we verified that GASC-exosomes profoundly modified the growth pattern of GSC as well as their motility and anchorage-independent growth [7]. Exosomes released by non-tumorigenic Wi38 fibroblast did not affect GSC, confirming the specific role of GASC-derived exosomes.
Interestingly, exosomes released both from HGG and LGG were able to increase the in vitro aggressiveness of glioblastoma cells, although those from LGG at a significant lower extent, thus suggesting that the degree of the tumor-supporting ability was proportional to the grade of malignancy of the tumor of origin.
At this regard, unpublished data from our laboratory seem to confirm the existence of differences in the miRNA content of exosomes released by GASC obtained from LGG and HGG, and even from LGG with a different prognosis [7].
Altogether, these results showed that functional features of tumor-supporting cells characterize GASC and that this effect can be at least in part attributable to the release of exosomes.
9.3.2.2 GASC Can Modulate the Adhesive Properties of Glioma Stem Cells
Active cell migration and invasion is a peculiar feature of glioma that renders this tumor able to migrate and infiltrate eloquent areas making difficult the achievement of a radical surgery. Migrating cancer cells undergo considerable molecular and cellular changes by remodeling their cytoskeleton and cell interactions with surrounding environment. Since we have seen that GASC can modify the motility of glioblastoma cells, we decided to get better insights into the interactions between GSC and GASC isolated from LGG and HGG [57]. By using time-lapse microscopy as well as atomic force microscopy and single cell force spectroscopy, we first demonstrated that, independently from the grade of the glioma of origin, GSC are softer than GASC, in agreement with their neoplastic features [57]. Subsequently we have shown that the adhesion strength of GSC on GASC appears to be significantly lower for cells derived from HGG with respect to those derived from LGG [57]. This is in line with the fact that HGG cells are characterized by a more infiltrative nature with respect to LGG. What was surprising is the fact that when GSC from HGG were cultured, in parallel, on GASC derived from LGG and HGG, they firmly adhere on GASC from LGG but not on those from HGG, suggesting that the grade of GASC plays an important role in modulating cancer cell adhesion, thus possibly affecting glioma cell migration, invasion and thus cancer aggressiveness [57].
Altogether these results provide evidence that investigating cell adhesion and elasticity of both tumor cells and tumor-supporting cells can open the way to novel diagnostic tool and suggest novel therapeutic targets.
9.3.3 GASC Are Endowed with a Prognostic Potential
Since GASC showed a state of activation that did not change upon in vitro expansion, and the extent of the tumor-supporting ability increased with the grade of gliomas, we wondered whether GASC features could predict the clinical behavior of the tumor (Fig. 9.3). We decided to focus our attention on LGG, trying to define new criteria to prognostically stratify this heterogeneous clinical entity [7].
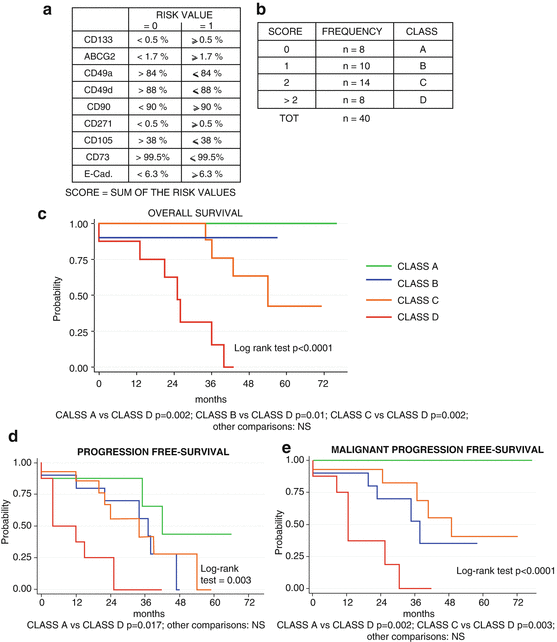

Fig. 9.3
Prognostic value of the GASC-based score in LGG patients. (a) Parameters and cut-off value used to define the GASC-related score. (b) Distribution of the LGG patients in four GASC-classes, according to the GASC-score value. (c–e) Kaplan-Meier curves showing OS (c), PFS (d), and MPFS (e) in LGG patients stratified according to the GASC-based score. Slightly modified by Bourkoula E, et al. Stem Cells. 2014;32:1239–53
The study was articulated in two parts. We first identified the GASC-features distinguishing LGG from HGG and we inserted them in a score that was finally tested for its capacity to prognostically stratify 40 LGG patients in terms of overall survival (OS), malignant progression free survival (MPFS) and progression free survival (PFS).
Comparing the features of GASC obtained from HGG and LGG and utilizing a ROC analysis, we selected nine parameters (among the surface proteins detected by flow-cytometry) significantly able to correctly classify the two groups and we determined the cut-off value able to discriminate the two populations (Fig. 9.3a). Of the nine parameters, five were more expressed (CD133, CD271, ABCG2, E-Cadherin, CD90) and four less expressed (CD49a, CD49d, CD105, CD73) in GASC from HGG with respect GASC from LGG.
We expressed the selected parameters as binary values creating a score based on the sum of these latter. This GASC-based score was indeed a number between 0 and 9, depending on the number of the GASC features similar to those obtained from HGG.
When the GASC-based score was assessed in a case study including 40 subsequent LGG-patients (median follow-up 36 months, range 13–76), we decided to stratify the patients in four classes (Fig. 9.3b). The 40 LGG were well characterized in terms of clinical (age, gender, extent of resection, subsequent adjuvant therapies) and histo-pathological (histotype, Ki67 expression, IDH1/2 gene mutation, 1p/19q co-deletion, MGMT-promoter-methylation) features.
Considering OS, PFS and MPFS, at the univariate analysis the GASC-based score was significantly associated with a worse prognosis, while the extent of tumor resection (EOR) and the presence of mutated IDH1 or IDH2 genes were protective factors. Importantly, the multivariate Cox analysis showed that the four classes’ GASC-based score was the only independent predictor of OS and MPFS, while EOR was the only independent predictor of PFS.
It is interesting to underline that the surface proteins included in the GASC score essentially belong to three classes: stem cell antigens (CD271, CD133 and ABCG2), adhesion proteins (CD49a, CD49d and E-Cadherin) and mesenchymal markers (CD90, CD73 and CD105). Specifically, GASC obtained from patients with poor prognosis were characterized by an increase in stem cell-related markers, a down-regulation in integrin expression and a variable modulation of mesenchymal markers. Moreover, these markers seemed to identify distinct sub-populations whose specific role in the natural history of glioma deserves future investigations.
Altogether, these results indicate that GASC are endowed with prognostic value, thus supporting the idea that this patient-based in vitro model could be helpful in a precision medicine approach aimed at customizing healthcare, with medical decisions, practices, and/or products being tailored to the individual patient.
9.3.4 GASC as a Potential Cellular Model for Precision Medicine
The precision medicine (PM) initiative sponsored by President Obama relies on the concept that prevention and treatment strategies must take individual variability into account [2, 69]. PM has become more realistic by the recent development of large-scale biologic databases (e.g. human genome sequence), powerful methods for characterizing patients (e.g. proteomics, metabolomics, genomics and diverse cellular assays), and computational tools for analyzing large sets of data [69]. Regarding cellular model, it is considered important by the National Cancer Institute (NCI), to develop new laboratory models of human cancer including human cancer cell lines and patient-derived tumor xenografts. These new models could help researchers in gaining new insights into tumor biology and better predicting patients’ responses to cancer treatment [69].
Since the difficulties in isolating GSC from LGG [70] and being GASC a strong predictor of LGG patients’ OS and MPFS outperforming the state-of-the-art prognostic factors [7], we have decided to explore this patient-based model to identify novel prognostic/predictive factors and to devise new therapeutic strategies aimed at targeting glioma by “curing” the tumor stroma (Fig. 9.4).
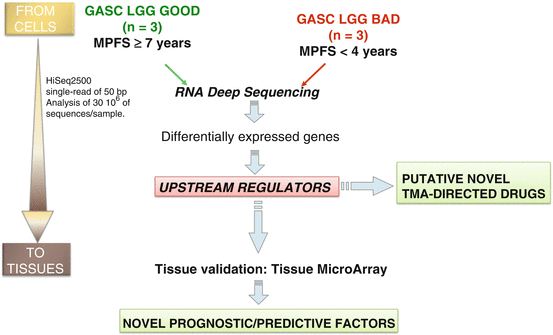

Fig. 9.4
Experimental approach using patient-derived GASC to identify novel prognostic/predictive factors and putative TME-directed drugs
At this regard, by using next generation sequencing techniques, we highlighted 81 genes and 15 miRNAs that distinguish GASC isolated from LGG with a good prognosis from those characterized by a rapid anaplastic transformation. Interestingly, most of the differentially expressed genes were related to an inflammatory and pro-angiogenic signature (unpublished data).
To facilitate the introduction of these new markers into the clinical routine, we started a project aimed at confirming the results, obtained at the cellular level, in a large case study including LGG tissues, by using immunohistochemistry, an ancillary technique currently used in all Pathology department. The proof of concept has been reached for one of these markers that resulted to be an independent predictor of both OS and MPFS (unpublished data).
Additionally, the above-mentioned analyses have also identified putative molecules and drugs that can act reverting the molecular pathways active in GASC obtained from LGG rapidly undergoing anaplastic transformation.
We intend now to provide evidence of whether, at least in vitro, it is possible to “cure” GASC by inhibiting their tumor-supporting function. Notably, stromal cells are optimal therapeutic targets for their genetic stability and lower potential to develop drug resistance [16, 41], although they can develop long-term resistance. However, the possibility to have cell cultures can help in developing in vitro model to explore these phenomena.
Altogether these results support the notion that GASC, being a patient-based model, can help in identifying biomarkers useful to predict prognosis and in devising novel therapeutic strategies aimed at interrupting the cross talk between tumor cells and their supporting stroma. Both properties of this cellular model can be instrumental at a precision medicine approach for LGG, an extremely heterogeneous clinical entity.
9.3.5 GASC: Still Open Questions
9.3.5.1 GASCs’ Origin
The presence of GASC raises issues regarding their origin in humans. In fact, their phenotype share many antigens with tumor cells, making difficult to unequivocally identify the in vivo counterpart of GASC in a tissue. Additionally, lineage-tracing experiments from the cell-of-origin cannot be done in humans [71]. However, some hypotheses on the GASC origin/in vivo counterpart can be done.
Regarding a possible fibroblast origin, in analogy to the tumor-associated fibroblast present in the epithelial tumors, it has to be underlined that there is a limited presence of fibroblasts in the CNS and their proliferation has never been described in the course of pathology [72]. Instead, the cell type that characterizes the CNS’ response to injury is represented by reactive astrocytes [73, 74]. Importantly, in murine models, non-neoplastic astrocytes could be converted, by the glioma microenvironment, into a reactive phenotype [75, 76] that could acquire stem cell features similar to GASC [77]. The activation of astrocytes seems to be mediated by an epithelial-to-mesenchymal like transition process [78], possibly regulated by the presence, in the tumor microenvironment of hypoxia and an increased myeloid cell number [79]. Unpublished data from our laboratory show that GSC-derived exosomes can indeed activate an epithelial-to-mesenchymal like transition process into cultured human astrocytes, which acquire stem cell properties.
Alternatively, GASC may derive from a population of perivascular mesenchymal stem cells endowed with both mesodermal and neuroectodermal differentiation capacities [80]. This opens the question regarding a possible connection between GASC and mesenchymal stem cells.
9.3.5.2 GASC and MSC
To date, the concept of “Mesenchymal stem cells” (MSC) is still a developing issue in terms of defining criteria, sites of origin, in vivo counterpart and function in normal and pathological conditions, including cancer. The demonstration of the existence of MSC into the brain opens the questions on the possible role of these cells in glioma and the relationship with GASC.
MSC: Definition Origin and Function
In 1960s Friedenstein first isolated from murine bone marrow a population of plastic-adherent, fibroblast-like cells able to form clonal colonies in vitro (colony-forming unit (CFU)—fibroblast). These cells, described as self-renewing non-hematopoietic bone marrow stromal stem cells (BMSC), were also capable of osteogenic differentiation in culture, could generate bone when implanted in ectopic locations in vivo and presented high self-renewal ability in serial implants [81]. Later on, Caplan coined the term “mesenchymal stem cells” and gave a full description of MSC features [82]. Currently, minimal criteria to define a cell as MSC have been described. Specifically, MSC must be plastic-adherent when maintained in standard culture conditions, must possess a specific surface phenotype and must differentiate into osteoblasts, adipocytes and chondroblasts in vitro [56, 83].
Regarding the site of origin, the concept that the bone marrow was the solely tissue hosting MSC has been discarded as this population was found in many tissues, such as adipose tissue, dental pulp, amniotic fluid, umbilical cord, muscles and many others, including brain [84, 85].
Considering the in vivo counterpart of MSC, lately researchers focused their attention on the concept that MSC home close to the vasculature, under the form of pericytes and adventitial cells. This new hypothesis has opened a new branch of research [86]. Bouacida et al. used in vivo and in vitro assays to support the idea that pericytes can be considered precursors of MSCs [87]. This theory might be able to explain the reason of the presence of MSCs in all tissues.
Moreover, Da Silva Meirelles et al. described some of the similarities between MSCs and pericytes, e.g. isolation methods, immunophenotype, clonogenicity, in vitro and in vivo multipotency, capacity to influence the immune system [88].
The great effort in defining criteria and protocol for MSC culture and characterization relies on their possible clinical use [86, 89]. The major clinical application of MSC are mainly attributed to their ability: to home to sites of injury when injected intravenously; to differentiate into various cell types [90]; to secrete multiple bioactive molecules capable of stimulating recovery of injured tissues and reducing inflammation and to perform immunomodulatory functions [89, 91, 92]. In fact, major applications are the treatment of the graft versus host disease and the regeneration of injured tissues, such as bone lesions and infarcted myocardium [89]. To date, clinicaltrials.gov reports more than 400 on-going trials using MSC in different disorders, especially in the field of bone regeneration and myocardial infarction.
An emerging role of MSC in cancer has been recently described.
MSC and Cancer
The role of MSC on tumor is still debated [93, 94]. MSC isolated from normal tissues present a tropism towards site of tumors and, while many studies have reported that these cells can support tumor progression and metastasis, others have shown that they can exert a tumor-suppressing function [93, 94]. Specifically, MSC can promote tumor growth by several mechanisms, including: differentiation into tumor-associated fibroblasts; suppression of the immune response; promotion of the angiogenesis; stimulation of epithelial-to-mesenchymal transition (EMT); contribution to the tumor microenvironment; inhibition of tumor cell apoptosis; and promotion of tumor metastasis [93, 94]. Conversely, MSC can act as tumor suppressors by: increasing inflammatory infiltration; inhibiting angiogenesis; suppressing Wnt signaling and AKT signaling, and inducing cell cycle arrest and apoptosis [93, 94].
Conversely, MSC isolated from the tumor, invariably present tumor-supporting functions [95].
Possibly, these apparently contrasting results can be explained considering the existence of a dynamic co-evolution of both tumor and stromal cells [53]. Therefore, MSC, once reached the site of tumor recruited by the inflammatory microenvironment, can modify and be modified by the tumor cells and the resulting function (tumor-suppressing or tumor-supporting) is initially the results of several factors, including the cytokines secreted by MSC and interactions between MSC, host immune cells and cancer cells [94]. However, with time, tumor MSC will be definitely “educated” by the tumor microenvironment to support tumor growth, progression and metastasis by recruiting additional immunosuppressive cells, enhancing the fraction of cancer stem cells in tumors, promoting EMT, and stimulating tumor angiogenesis [95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree