Difficult Pain Syndromes: Bone Pain, Visceral Pain, Neuropathic Pain
Marco Pappagallo
Lauren Shaiova
Eugene Perlov
Helena Knotkova
Nociception is what occurs physiologically in our bodies during the activation and sensitization of tissue nociceptors, also known as A-delta and C nerve fibers. Pain corresponds to our awareness of nociception and has been defined as “an unpleasant sensory and emotional experience associated with tissue damage or described in terms of such damage” (1).
In the clinical setting, pain may occur as a response to a noxious event in the tissue, for example, tissue inflammation due to a burn injury, or as a response to an abnormal pathologic process occurring within the nervous system pain pathways. In the first case, the pain signal presumably originates from “healthy” tissue nociceptors activated or sensitized by the local release of algogenic substances (e.g., protons, prostaglandins, bradykinin, adenosine, cytokines, etc.). This type of pain is called nociceptive. In the second case, the pain signal is generated ectopically by abnormal peripheral nerve fibers involved in pain transmission and/or by abnormal pain circuits in the central nervous system (CNS); this type of pain has been called neuropathic. However, the separation between nociceptive and neuropathic pain states is often blurred. Indeed, as discussed in the subsequent text, neuropathic pain may arise from inflammation (i.e., inflammatory neuropathic pain) (2). Inflammatory and neuropathic mechanisms may be present at the same time or at different times in patients who have been diagnosed with cancer pain syndromes of bone or visceral origin. In fact, cancer pain, whether arising from viscera, bone, or any other somatic structure, is more often than commonly thought the result of a mixture of pain mechanisms. When cancer pain becomes a clinical challenge to treatment, it has been labeled as a difficult pain syndrome.
Difficult Pain Syndromes: Peripheral and Central Mechanisms
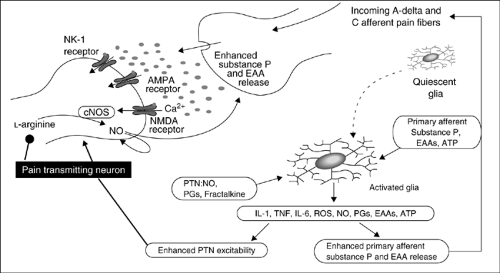
The pain signal is transmitted from the peripheral nociceptors, through the dorsal horn of the spinal cord and the thalamus, up to the cortex. In the periphery, nociceptors can be activated by chemical products of tissue damage and inflammation, which include prostanoids, serotonin, bradykinin, cytokines, adenosine, adenosine-5′-triphosphate (ATP), histamine, protons, free radicals, and growth factors. These agents can activate afferent fibers or sensitize them to a range of mechanical, thermal, and chemical stimuli. Notably, a proportion of the afferent fibers that are normally unresponsive to noxious stimuli (“silent” or “sleeping” nociceptors) can be “awakened” by inflammatory chemicals and be stimulated to contribute to pain and hyperalgesia. The products of tissue damage and inflammation interact with receptors located on the A-delta fibers and C fibers to initiate membrane excitability and intracellular transcriptional changes.
Most neuropathic pain conditions develop after partial injuries to the peripheral nervous system (PNS). For example, as observed in animal models of partial nerve injury, both injured and uninjured primary sensory neurons acquire the ability to express genes de novo and, therefore, change their phenotype (phenotypic shift). Nerve endings develop sensitivity to a number of factors, such as prostanoids and cytokines [e.g., tumor necrosis factor-α (TNF-α)] (3, 4, 5, 6). One example is the upregulation or induction of catecholamine receptors in undamaged nociceptors; in this condition, nociceptors are activated by noradrenaline, and the resulting neuropathic pain has been called sympathetically maintained pain (SMP) (7, 8). Reversal of the phenotypic shift is associated with the reduction of neuropathic pain (9).
Recent findings suggest that during cancer (and other pathologic inflammatory conditions), a number of diffusible factors might be involved in causing a “neuropathic spin” in the cancer-related pain state. Tissue-related growth factors [e.g., nerve growth factor (NGF)] in combination with specific proinflammatory cytokines (e.g., TNF-α, interleukin-1β (10)) might sensitize nociceptors and generate ectopic and spontaneous activity in tissue nociceptors. In these instances, pain caused by cancer could be classified more properly as inflammatory neuropathic pain. There is considerable hope that the identification of the diffusible factors causing altered gene expression in the dorsal root ganglia (DRG) sensory neurons will direct research to discover more effective treatments. Early and aggressive pain interventions and the use of specific therapies that disengage gene expression might be sufficient to uncouple the phenotypic shift and reverse a difficult pain syndrome into an easy-to-treat condition.
Table 2.1 Development of Pathologic Pain: Relevant Peripheral Nervous System Factors | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Peripheral Mechanisms of Pathologic Pain
In the PNS, several elements of the cellular “machinery” thought to be relevant to the development of pathologic pain have been identified as potential targets for analgesic drugs. These PNS targets are summarized in Table 2.1.
Central Mechanisms of Pathologic Pain
Neuropathic Pain
Clinical Findings and Diagnosis
The clinical interview of a patient with cancer pain should focus on questions about onset, duration, progression, and nature of complaints suggestive of neurologic deficits (e.g., persistent numbness in a body area, or limb weakness, such as tripping episodes, the progressive inability to open jars, etc.), as well as complaints suggestive of sensory dysfunction (e.g., touch-evoked pain, intermittent abnormal sensations, spontaneous burning, and shooting pains). Notably, patients may report only sensory symptoms and have no neurologic deficits.
Patients with neuropathic pain may present with some or all of the following abnormal sensory symptoms and signs:
Paresthesias Spontaneous, intermittent, painless, abnormal sensations
Dysesthesias Spontaneous or evoked unpleasant sensations, such as annoying sensations elicited by cold stimuli or pinprick testing
Allodynia Pain elicited by nonnoxious stimuli (i.e., clothing, air movement, tactile stimuli) when applied to the symptomatic cutaneous area; allodynia may be mechanical (static, e.g., induced by application of a light pressure, or dynamic, e.g., induced by moving a soft brush) or thermal (e.g., induced by a nonpainful cold or warm stimulus)
Hyperalgesia An exaggerated pain response to a mildly noxious (mechanical or thermal) stimulus applied to the symptomatic area
Hyperpathia A delayed and explosive pain response to a stimulus applied to the symptomatic area
Allodynia, hyperalgesia, and hyperpathia represent positive abnormal findings, as opposed to the negative findings of the neurologic sensory examination, that is, hypesthesia and anesthesia. Heat hyperalgesia and deep mechanical allodynia (i.e., tenderness on soft tissue palpation) are findings commonly present at the cutaneous epicenter of an inflammatory pain generator, also known as the zone of primary hyperalgesia. These findings are indicative of PNS sensitization and are related to a local inflammatory state. On the other hand, the skin surrounding the site of inflammation, also known as the zone of secondary hyperalgesia, may present the finding of mechanical allodynia, which can be elicited, for example, by stroking the area with a soft brush. Secondary hyperalgesia is indicative of CNS sensitization. Patients affected by SMP typically complain of cold allodynia/hyperalgesia. This is assessed by providing a cold stimulus, such as placing a cold metallic tuning fork, to the painful region for a few seconds.
Clinical and research tools to assess and measure the intensity and quality of neuropathic pain include the Brief Pain Inventory (BPI) and the Neuropathic Pain Scale (NPS) (41). The BPI is a well-validated instrument that consists of 15 items asking the patient about average pain, worst pain in the past week, whether the patient has received relief from pain treatment, and whether the pain has interfered with daily activities (42). The NPS is a self-report scale for measuring neuropathic pain. It consists of 12 distinct questions, which ask about intensity and quality of the patient’s pain. In validation studies, it has been found to have a good predictive power in discriminating between major subgroups of patients with neuropathic pain (42).
Table 2.3 (43) lists the most common neuropathic pain syndromes that have been reported in association with cancer. Neuropathy may result from one or more cancer-related mechanisms (44), for example, compression, mechanical traction, inflammation, or infiltration of nerve trunks or plexi caused by the progression of the primary cancer or by metastatic disease affecting bone or soft tissues. Head and neck cancer and skull-based tumors can cause painful cranial neuropathies by direct nerve compression. Salivary gland cancers may cause painful facial neuropathies. Breast or lung cancer can infiltrate the brachial plexus and cause painful plexitis. Pelvic or retroperitoneal cancer may invade the lumbosacral plexus. If the meninges are affected (meningeal carcinomatosis), the involvement of adjacent roots, spinal nerves, and plexi can occur. Metastatic disease or lymphoma can cause meningeal carcinomatosis and affect multiple spinal roots. Peripheral neuropathies with pain and dysesthesia may also be observed in the presence of lymphomas. Acute inflammatory demyelinating polyneuropathy of the Guillain-Barré syndrome type may occur with lymphomas, particularly Hodgkin’s disease.
Antineoplastic therapeutic agents such as cis-platinum, taxoids, and vincristine may cause painful neuropathies. Postradiation plexopathies may arise when >60 Gy (6000 rad)
of irradiation are given to the patient. Surgical resection of cancers may result in traumatic injuries to peripheral nerves, with the development of painful neuromas. For example, postthoracotomy pain can be caused by injury to the intercostal nerves, and postmastectomy pain may arise through injury to the intercostobrachial nerve.
of irradiation are given to the patient. Surgical resection of cancers may result in traumatic injuries to peripheral nerves, with the development of painful neuromas. For example, postthoracotomy pain can be caused by injury to the intercostal nerves, and postmastectomy pain may arise through injury to the intercostobrachial nerve.
Table 2.2 Development of Pathologic Pain: Relevant Central Nervous System Factors | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Table 2.3 Classification of Neuropathic Cancer Pain Syndromes | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
Compression or entrapment neuropathies occur in the presence of cachexia; for example, patients with cancer who have lost substantial fat and muscle body weight are prone to develop peroneal neuropathies.
Paraneoplastic autoimmune syndromes due to antineuronal antibodies may present as painful neuropathies. Patients who complain of burning dysesthesias in their feet, hands, and face (in the setting of diagnosed or undiagnosed carcinoma) may have antineuronal nuclear antibodies type 1 (ANNA-1), also known as anti-Hu. Most patients who present with sensory neuronopathy and small cell carcinoma of the lung have significantly elevated titers of anti-Hu. All patients with burning dysesthesias of face, hands, and legs and positive titers for anti-Hu should undergo a computed tomography (CT) or magnetic resonance imaging (MRI) of the chest. In fact, small cell carcinoma of the lung may remain undetected by plain chest x-ray. In any case, anti-Hu positivity should prompt a careful search for malignancy, especially for a small cell carcinoma of the lung. Painful dysesthesias develop first in one limb and then progress to involve other limbs, face, scalp, and trunk over weeks or months. In these patients, deep tendon reflexes are reduced or absent and muscle strength is preserved. Patients may be disabled in their ambulation because of the sensory ataxia that is often associated with the painful symptoms.
Therapeutic Interventions for Neuropathic Pain
Management of severe neuropathic pain can be a challenge, and a combination of therapies employing agents from a variety of pharmacologic classes and pain procedures represent the contemporary standard approach (Table 2.4). Treatment includes a wide range of modalities, ranging from opioid and nonopioid analgesics to implantable devices and surgery.
Antiepileptic Drugs
Antiepileptic drugs (AEDs) are becoming the most promising agents for the management of neuropathic pain. The gabapentinoid anticonvulsants gabapentin and pregabalin have both established efficacy in treating neuropathic pain. In May 2002, gabapentin gained U.S. Food and Drug Administration (FDA) approval for the treatment of postherpetic neuralgia (PHN), a state characterized by allodynia and burning pain. However, gabapentin is also known to be effective in treating neuropathic pain from diabetic neuropathy, a state predominantly characterized by spontaneous burning pain (45, 46, 47). In December 2004, the gabapentin analog pregabalin gained FDA approval for the treatment of PHN and painful diabetic neuropathy. Gabapentinoids act on neither γ-aminobutyric acid (GABA) receptors nor sodium channels. Recent evidence suggests that gabapentin and pregabalin may modulate the cellular calcium influx into nociceptive neurons by binding to voltage-gated calcium channels, in particular to the α-2-Δ subunit of the channel (48). Trigeminal neuralgia
(a neuropathic condition characterized by brief excruciating lancinating pains) responds extremely well to carbamazepine, while another AED, lamotrigine, has shown some efficacy in treating carbamazepine-resistant trigeminal neuralgia (49). Topiramate has been anecdotally used in the treatment of complex regional pain syndrome (CRPS) type 1 (50). Several new AEDs (e.g., levetiracetam, zonisamide, oxcarbazepine, and tiagabine) have become available for medical use, and some of these, along with topiramate, may have analgesic effect in primary headache and perhaps in neuropathic pain (51, 52). Interestingly, in a recent randomized, double-blind, active placebo-controlled, crossover trial, patients with neuropathic pain received lorazepam (active placebo), controlled-release morphine, gabapentin, and a combination of gabapentin and morphine, each treatment given orally for 5 weeks. The study indicated that the best analgesia was obtained from the gabapentin–morphine combination, with each medication given at a lower dose when given as a combination than when given as a single agent (53).
(a neuropathic condition characterized by brief excruciating lancinating pains) responds extremely well to carbamazepine, while another AED, lamotrigine, has shown some efficacy in treating carbamazepine-resistant trigeminal neuralgia (49). Topiramate has been anecdotally used in the treatment of complex regional pain syndrome (CRPS) type 1 (50). Several new AEDs (e.g., levetiracetam, zonisamide, oxcarbazepine, and tiagabine) have become available for medical use, and some of these, along with topiramate, may have analgesic effect in primary headache and perhaps in neuropathic pain (51, 52). Interestingly, in a recent randomized, double-blind, active placebo-controlled, crossover trial, patients with neuropathic pain received lorazepam (active placebo), controlled-release morphine, gabapentin, and a combination of gabapentin and morphine, each treatment given orally for 5 weeks. The study indicated that the best analgesia was obtained from the gabapentin–morphine combination, with each medication given at a lower dose when given as a combination than when given as a single agent (53).
Table 2.4 Analgesic Algorithm For Neuropathic Pain—Steps of Intervention | |||||
|---|---|---|---|---|---|
|
Opioids
Opioids are currently the most potent and effective analgesics used to treat acute and chronic pain, and, as such, they have been prescribed to patients suffering from intractable pain. Morphine, a μ agonist, represents the mainstay for the treatment of moderate to severe nociceptive cancer pain (54). Long considered to be ineffective for neuropathic pain, opioids have demonstrated efficacy in several recent clinical trials (55, 56, 57, 58, 59, 60). A double-blind, placebo-controlled, crossover trial (57) in which 76 patients with PHN received opioids (e.g., controlled-release morphine or methadone), tricyclic antidepressants (TCAs) (e.g., amitriptyline or nortriptyline), and placebo found that both opioids and TCAs provided significantly better pain relief than placebo. Among patients completing the study, most preferred opioids (50%) to TCAs (30%; p = .02). The results indicate that opioids are as effective as TCAs in the treatment of PHN.
The analgesic action of the pure opioid agonists (e.g., morphine, methadone, fentanyl, oxycodone, hydromorphone, etc.) is well known and utilized clinically. Among all the analgesic medications currently available, the most powerful and effective drugs are still the agents acting on the μ-, κ-, and Δ-opioid receptors. Opioid receptors are located not only in the CNS (primarily in the dorsal horn) but also peripherally on the nociceptors. Opioids may have a relevant peripheral analgesic effect during painful inflammatory states (61).
The pure opioid agonists are the mainstay for the treatment of severe disabling pain. The treatment of chronic pain may rely on the use of long-acting agents (i.e., methadone, levorphanol) or controlled-release preparations of morphine, fentanyl, and oxycodone. Among the pure opioid agonists, methadone has peculiar properties. It has an intrinsic N-methyl-D-aspartate (NMDA) receptor antagonistic effect, which may add adjuvant analgesic effect in case of neuropathic pain (see subsequent text). Interestingly, recent animal studies suggest that the addition of an extremely low dose of an opioid receptor antagonist (e.g., naltrexone) to morphine in a ratio of 1:1000 may enhance the analgesic efficacy of the opioid agonists (62). Tramadol is an analgesic agent with a weak μ-opioid agonistic effect. Its potency is comparable to that of a codeine–acetaminophen preparation. Notably, in controlled trials, tramadol has shown efficacy in the treatment of neuropathic pain (62, 63, 64).
Clinicians should be careful during opioid titration because the requirement for neuropathic pain may be high. The opioid dose should be increased until analgesia is achieved or till side effects become intolerable. Common side effects are constipation, sedation, pruritus, and nausea/vomiting. Rarely, frank confusion may develop. Except for constipation, tolerance occurs for most of the opioid-related side effects (e.g., nausea, vomiting, respiratory depression, and drowsiness). The most feared complication of respiratory depression is rare, especially in patients who are somewhat tolerant to opioids. Unlike anti-inflammatory drugs, opioid agonists have no true “ceiling dose” for analgesia and do not cause direct organ damage. Side effects can often be managed with additional pharmacotherapy, and the clinician may choose to treat the side effects and continue the opioid dose, or “rotate” to another opioid. When converting to another opioid, it is wise to refer to an opioid conversion table or a similar reference and reduce the dose by 50% to avoid incomplete cross-tolerance. Opioid titration and opioid rotation are essential concepts in the management of neuropathic pain. To determine adequate opioid responsiveness, a careful titration of the opioid dose is necessary. However, the development of tolerance to opioid side effects, degree of analgesia, and the development of analgesic tolerance are extremely variable among patients with pain receiving these medications. If severe pain persists or side effects become intolerable during the initial drug trial, trials of different opioids (i.e., opioid rotation) are recommended. Studies indicate that patients on a stable opioid regimen do not report significant impairment in their driving ability, attention, mood, and general cognitive functioning (65).
Antidepressants
Antidepressants also play an important role in the treatment of chronic pain. TCAs, such as amitriptyline, nortriptyline, and desipramine (66), have established efficacy in the treatment of neuropathic pain. They have been used successfully for painful diabetic neuropathy and PHN and provide pain relief in nondepressed patients affected by neuropathic pain. Notably, TCAs such as amitriptyline, doxepin, and imipramine have been found to have potent local anesthetic properties. Amitriptyline appears to be more potent than bupivacaine as a sodium channel blocker (67). TCAs frequently have poorly tolerated adverse effects, including cardiotoxicity, confusion, urinary retention, orthostatic hypotension, nightmares, weight gain, drowsiness, dry mouth, and constipation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree