Diarrhea, Malabsorption, and Constipation
Sebastiano Mercadante
Diarrhea
Diarrhea is a common complication in the cancer population, occurring in 5–10% of patients with advanced disease. Women are more likely to have diarrhea than men after excluding gender-specific cancers (1). Diarrhea is also included among the top ten consequences of adverse drug reactions in hospitalized patients with cancer (2). The consequences of diarrhea can be troublesome, and include loss of water, electrolytes, and albumin, failure to reach nutritional goals, declining immune function, and the risk of bedsores or systemic infection. Diarrhea also brings additional work for the nursing staff or family who have to prevent maceration and bedsores. Moreover, losses of comfort and dignity have to be considered. Severe diarrhea, other than being debilitating, is a costly complication of chemotherapy in colorectal cancer. In patients with colorectal cancer receiving chemotherapy, the median length of hospital stay due to diarrhea was 8 days, translating to a mean cost of $8230 per patient (3).
Although a practical definition is lacking, diarrhea is commonly diagnosed when an abnormal increase in daily stool weight, water content, and frequency, whether or not accompanied by urgency, perianal discomfort, or incontinence, is present as a consequence of incomplete absorption of electrolytes and water from luminal content (4). Common causes among patients with cancer are listed in Table 15.1.
Mechanisms
From the physiopathologic point of view, different mechanisms may produce diarrhea, although it is quite difficult in certain clinical conditions to distinguish among mechanisms that frequently overlap.
Osmotic Diarrhea
The ingestion of a poorly absorbable solute modifies the osmolarity of the luminal content and induces osmotic diarrhea. The proximal small bowel is highly permeable to water; sodium and water influx across the duodenum rapidly adjusts the osmolarity of luminal fluid toward that of plasma, secreting water even after the osmolarity values between luminal contents and plasma are similar. On the contrary, the mucosa of the ileum and colon has a low permeability to sodium and solutes. However, there is an efficient active ion transport mechanism that allows the reabsorption of electrolytes and water even against electrochemical gradients (4).
Carbohydrates and proteins are fermented by colonic bacteria and salvaged as short-chain fatty acids and gases. Anaerobic colonic flora are necessary for the fermentation of fiber into short-chain fatty acids and constitute the bulk of fecal mass.
When large amounts of lactulose, an unabsorbable sugar in the small intestine, are ingested, the protective role of colonic bacteria may be exhausted, resulting in severe diarrhea, proportional to the osmotic force of the malabsorbed saccharide (5). It is characterized by an osmotic gap in stool analysis equivalent to the concentration of the osmotically active agents in fecal fluid that cause diarrhea (4). Similarly, carbohydrate malabsorption may induce osmotic diarrhea, which is characterized by a low stool pH, because of the presence of short-chain fatty acids, a high content of carbohydrates, high stool osmolarity, and flatulence. Moreover, reversible chemotherapy-related hypolactasia and lactose intolerance are not infrequent in patients treated with 5-fluorouracil (5-FU)–based adjuvant chemotherapy for colorectal cancer. Avoidance of lactose during chemotherapy may improve treatment tolerability in these patients (6).
The ingestion of other substances, such as magnesium, sulfate, and poorly absorbed salts may produce osmotic diarrhea. However, there will be a normal pH, unlike in carbohydrate-induced diarrhea. In the perioperative period, massive antibiotic therapy is able to suppress normal colonic metabolism, thereby resulting in diarrhea (5). Osmotic diarrhea commonly subsides as the patient discontinues the poorly absorbable agents.
Secretory Diarrhea
Secretory diarrhea is rarely present as the sole mechanism and is often associated with other mechanisms (5). This kind of diarrhea is associated with an abnormal ion transport in intestinal epithelial cells, with a reduction in absorptive function or increase in the secretion of epithelial cells.
Table 15.1 Causes of Diarrhea in Patients With Cancer | ||||||
|---|---|---|---|---|---|---|
|
Unlike in osmotic diarrhea, the anionic gap is small and eating does not markedly increase stool volume. Moreover, diarrhea usually persists despite fasting.
Many factors may affect ion transport in the epithelial cells of the gut. These include bacterial toxins, intraluminal secretagogues (such as bile acids or laxatives) or circulating secretagogues (such as various hormones, drugs, and poisons). Moreover, other medical problems that compromise regulation of intestinal function or reduce absorptive surface area (by disease or resection) can induce secretory diarrhea (7).
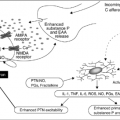
Endocrine tumors may cause diarrhea through the release of secretagogue transmitters (8). Diarrhea is a common manifestation of a carcinoid syndrome, occurring in approximately 70% of patients, and seems to be mediated by the release of serotonin and substance P. In the Zollinger-Ellison syndrome, secretory diarrhea is the consequence of gastric hypersecretion caused by a high concentration of circulating gastrin, overwhelming the intestinal absorptive capacity. In a medullary carcinoma of the thyroid, circulating calcitonin is the major mediator of intestinal secretion (4).
Malabsorption due to different mechanisms may equally produce diarrhea (9) (see section on Malabsorption).
Cancer treatment–related diarrhea caused by acute and chemotherapeutic agents such as fluoropyrimidines, paclitaxel, and irinotecan, (3) and graft versus host disease (GVHD) (10) significantly affects morbidity and mortality. Diarrhea is a significant consequence of colorectal chemotherapy, with most patients experiencing grade 3 or 4 diarrhea. Severe diarrhea developed after the first cycle of chemotherapy in 58% of the patients and contributed to a dose reduction, change, or discontinuation of chemotherapy in 9.5, 15.9 and 34.2% of patients, respectively (3). These agents cause acute and chronic damage to the intestinal mucosa, necrosis, and extensive inflammation of the bowel wall. Mucosal and submucosal factors, produced directly or indirectly by the inflamed intestine, stimulate secretion of intestinal fluid and electrolytes. Delayed-onset diarrhea, with an incidence of 20–35% at grade 3–4 level is one of the most important causes of dose-limiting toxicity of irinotecan (11). Similar anatomic changes have been observed in patients with GVHD diarrhea, as well as with radiation enteritis (12). The toxicity grades of these agents seem to depend on individual genotypes (13).
On the other hand, intestinal mucositis also increases the risk of superinfection by opportunistic pathogens such as Clostridium difficile, Clostridium perfringens, Bacillus cereus, Giardia lamblia, Cryptosporidium, Salmonella, Shigella, Campylobacter, and Rotavirus, particularly in patients who may be neutropenic or immunosuppressed. Bacterial enterotoxins or other infective agents induce secretion probably by a local nervous reflex mediated by enteroendocrine cells or inflammation (4). The incidence of C. difficile–induced diarrhea is very high—2.2% in patients receiving standard-dose regimens and 20% in patients receiving high-dose regimens (14).
The use of long-term antibiotics is also associated with diarrhea in patients who recently underwent surgery or are immunocompromised. Agents more frequently causing diarrhea include ampicillin, clindamycin, or cephalosporins, because of the disruption of the normal flora and facilitation of the overgrowth of pathogens. C. difficile, an anaerobic organism producing an enteric toxin, induces pseudomembranous enterocolitis, which presents as a severe microbial diarrhea. Other infectious agents include C. perfringens, Staphylococcus aureus, Klebsiella oxytoca, candida species, and salmonella species (15).
Finally, many drugs may cause diarrhea. Diuretics, caffeine, theophylline, antacids, antibiotics, and poorly absorbable laxative agents and osmotically active solutes, often chronically administered in a palliative care setting, likely produce reflex nervous secretion or directly activate secretory cellular mechanism (4).
Deranged Motility
Defective motility may reduce the contact time between luminal contents and epithelial cells. This commonly occurs in patients with cancer with postsurgical disorders, such as postgastrectomy dumping syndrome, postvagotomy, ileocecal valve resection or neoplastic and chronic diseases, such as malignant carcinoid syndrome, medullary carcinoma of the thyroid, and diabetes. The mechanism by which diabetic neuropathy causes dysmotility is attributed to a sympathetic denervation of the bowel with a prevalence of cholinergic innervation (15). Similarly, procedures such as celiac plexus block produce a sympathetic denervation of the bowel, which may leave a cholinergic innervation unopposed, leading to an increase in intestinal motility and diarrhea, until adaptation mechanisms develop (4).
Spinal cord damage may reduce intestinal mobility favoring bacterial overgrowth, which induces a deconjugation of bile acids in the small bowel and thereby causes diarrhea and steatorrhea. Diarrhea secondary to dysmotility disorders commonly subsides after a 1–2-day fast, determining a small stool volume and an osmolality in the range of 250–300 mOsm.
Assessment
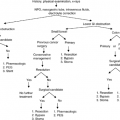
The patient should be questioned in detail about dietary habits, the use of drugs, and previous surgery. Frequency, amounts, and consistency of the stools should be carefully obtained. When the stools are consistently large, light in color, watery or greasy, free of blood, or contain undigested food particles, the underlying disorder is likely to be in the small bowel or the proximal colon. Indeed, small stool diarrhea, in which frequent but small quantities of feces that are dark in color and often contain mucus or blood pass in spite of a sense of urgency, is associated with a disorder of the left colon or rectum (4). Widespread inflammation may simultaneously produce both patterns of diarrhea, confirmed by the passage of nonbloody diarrheal fluid, pus, or exudates. Other useful information includes fecal incontinence, change in stool caliber, rectal bleeding, and small, frequent, but otherwise normal stools.
Timing and spontaneous recovery are also important. Although osmotic diarrhea typically stops or reduces after fasting or stopping the drug previously used, secretory diarrhea persists in spite of fasting. Chemotherapy-induced diarrhea typically occurs 2–14 days after therapy. Radiation colitis is probable in patients who have recently received pelvic radiation for malignancies of the urogenital tract and of the prostate.
A physical examination should precede any further investigation. Signs of anemia, fever, postural hypotension, lymphadenopathy, neuropathy, hepatosplenomegaly, ascites, gaseous abdominal distention or lymphadenopathy, reduced anal sphincter tone, a rectal mass or impaction, and deterioration of nutritional status are of paramount importance in defining the type of diarrhea. Some etiologies may have a typical clinical pattern. For example, carbohydrate malabsorption is typically associated with excessive flatus and mushy stools, whereas intermittent diarrhea and constipation are frequent in diabetic neuropathy, as well as in irritable bowel syndrome or subobstructive disorders. Autonomic neuropathy or anal sphincter dysfunction may be characterized by nocturnal diarrhea and fecal soiling. Alternating diarrhea and constipation suggests fixed colonic obstruction. Fecal impaction may cause apparent diarrhea because only liquids pass a partial obstruction. Symptoms of dumping syndrome after gastric
surgery, such as early nausea, abdominal distention, weakness, and diarrhea after a meal followed by hypoglycemia, sweating, dizziness, and tachycardia, are typical. Secretory diarrhea combined with upper gastrointestinal (GI) symptoms caused by refractory peptic ulcer disease is suggestive of a gastrin-secreting tumor. High circulating serotonin levels in carcinoid syndrome cause other effects besides diarrhea, including hypotension, sweating, flushing, palpitation, and wheeziness (4, 15). The association of heat intolerance, palpitations, and weight loss suggests possible hyperthyroidism. Intestinal dysmotility or bacterial overgrowth due to diabetes, neoplastic conditions, or postoperative conditions should be suspected, excluding other causes.
surgery, such as early nausea, abdominal distention, weakness, and diarrhea after a meal followed by hypoglycemia, sweating, dizziness, and tachycardia, are typical. Secretory diarrhea combined with upper gastrointestinal (GI) symptoms caused by refractory peptic ulcer disease is suggestive of a gastrin-secreting tumor. High circulating serotonin levels in carcinoid syndrome cause other effects besides diarrhea, including hypotension, sweating, flushing, palpitation, and wheeziness (4, 15). The association of heat intolerance, palpitations, and weight loss suggests possible hyperthyroidism. Intestinal dysmotility or bacterial overgrowth due to diabetes, neoplastic conditions, or postoperative conditions should be suspected, excluding other causes.
Chronic bowel ischemia should be considered in elderly patients with the clinical features of diffuse atherosclerotic disease. Rectal examination and abdominal palpation should be performed to look for fecal masses and to exclude fecal impaction and intestinal obstruction, as well as for perianal fistula or abscess. Rectal involvement is probable in the presence of tenesmus, commonly defined as the passing of a little or no stool in spite of a sense of rectal urgency.
Of course, the site of neoplasm and metastases is of paramount importance. An abdominal x-ray will help the diagnosis. The location of the tumor will be verified by computed tomography scan, magnetic resonance imaging, angiography, or laparoscopy.
Laboratory findings should complete the investigation. If feasible, collected diarrhea stool specimen should be submitted for qualitative study. A positive finding in either the stool guaiac or leukocyte test leads to a suspicion of an exudative mechanism, as in radiation colitis, colonic neoplasm, or infective diarrhea. Stool cultures for bacterial, fungal, and viral pathogens, as well as a formal evaluation of the GI tract should complete the initial assessment (12). Gram stain of the stool can diagnose the presence of Staphylococcus, Campylobacter, or Candida infection. Multiple stool cultures should be obtained from patients with secretory diarrhea to rule out microorganisms producing enterotoxins that stimulate intestinal secretion. The presence of a microorganism in the stools is diagnostic.
An anion gap of >50 mmol per L due to a reduction of stool content in sodium and potassium suggests an osmotic diarrhea, whereas lower values (<50 mmol per L) indicate a secretory diarrhea due to active secretion of salts and water.
Treatment
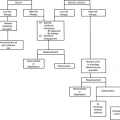
As a general rule, current medication should be revised, considering the use of laxatives, antacids, theophylline preparations, central nervous system drugs, antiarrhythmics, and antibiotics. Dietary advice may be helpful in some circumstances, although difficult to follow by most patients with cancer. A gluten-free diet can reduce abdominal cramping and frequency of bowel movement in the presence of intestinal fermentation with bowel distention. Binders of osmotically active substances (kaolin-pectin) give a thicker consistency to loose stools, producing a viscous, colloidal, absorbent solution, but their antidiarrheal effectiveness is disputable. Apples without the peel are particularly rich in pectin. Other dietary advice include avoiding cold meals, milk, vegetables rich in fibers, fatty meat and fish, coffee, and alcohol.
As diarrhea is associated with the occurrence of dehydration, the patient should be rehydrated, possibly by oral solutions containing glucose, electrolytes, and water. However, clinical signs of dehydration, such as orthostatic hypotension, decreased skin turgor, and a dry mouth, suggest the need for intensive hydration by intravenous route, especially in patients suffering from nausea and vomiting, in whom oral therapy is ineffective (4).
Table 15.2 Etiology-Based Treatment | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Considering the different mechanisms involved in determining diarrhea in patients with cancer, there are no broadly accepted treatment protocols. Particular strategies have been anecdotally suggested for specific etiologies (Table 15.2). Cholestyramine, aspirin, and sucralfate have been favorably used in radiation-induced diarrhea (16). Silicate smectite has proved a promising drug in the prophylaxis of radiotherapy-induced diarrhea, particularly in patients with a low, irradiated, small bowel volume (17). From recent investigations it seems that the best treatment would be prevention. In gynecologic malignancies, prior operation with low pelvic fields and prior operation with small volume were significantly protective factors for overall diarrhea. Conversely, large volume was a significant factor of overall and moderate to severe diarrhea in patients with large-field operations (18). Hypofractionated and accelerated radiotherapy for prostate cancer were supported with high-dose daily amifostine (1000 mg s.c.) to protect normal tissues against early and late effects, and produced only grade 0–1 cystitis or diarrhea (5/7 grade 0). Amifostine tolerance was excellent (19).
Steroids may exert a positive effect on several conditions associated with diarrhea, including secretory diarrhea, intestinal pseudo-obstruction, radiation-induced enteritis, endocrine tumors due to anti-inflammatory effects, and the capability of reducing the release and effect of inflammation mediators, and promoting salts and water absorption. They are also included in the pharmacologic approach to GVHD-induced diarrhea (12). Budesonide, a topically active steroid, demonstrated a substantial activity in irinotecan and 5-FU–induced diarrhea after failure of loperamide treatment (20).
Antibiotics, such as norfloxacin and amoxicillin-clavulanic acid, are effective in the treatment of bacterial overgrowth–related diarrhea (21). On the contrary, antibiotic-associated diarrhea (pseudomembranous enterocolitis) requires the discontinuation of antibiotics and the starting of either metronidazole or vancomycin (22). Bismuth subsalicylate in doses of 30–60 mL every 30 minutes for eight doses may bring mild symptomatic relief in patients with acute infectious diarrhea with an unknown effect (4). Probiotic bacteria (i.e., live bacteria that survive passage through the GI tract) may
have beneficial effects on the host. In a recent review, probiotic bacteria consistently shortened the diarrheal phase of rotavirus infection, although the evidence for other viral or bacterial infections was less strong (23).
have beneficial effects on the host. In a recent review, probiotic bacteria consistently shortened the diarrheal phase of rotavirus infection, although the evidence for other viral or bacterial infections was less strong (23).
Alkalization of the intestinal tract by oral administration of sodium bicarbonate has been reported to be a promising method for preventing delayed diarrhea, a dose-limiting toxicity in patients receiving chemotherapy with irinotecan, without decreasing the blood levels of irinotecan and its active metabolites, thereby improving the tolerability of long-term chemotherapy without reducing the efficacy (24). An oral adsorbent (2g Kremezin × three times) has been shown to decrease the number of bowel motions, without decreasing the plasma clearance of irinotecan much (25). Activated charcoal, given the evening before the irinotecan dose and then t.i.d. for 48 hours after the dose, reduced irinotecan-induced diarrhea and optimized its dose-intensity, possibly by adsorbing free lumenal SN-38, the irinotecan-active moiety that has a direct effect on mucosal topoisomerase-I (11). Broad-spectrum antibiotics may influence the intestinal toxicity of irinotecan. Although neomycin had no effect on the systemic exposure of irinotecan and its major metabolites, it changed fecal β-glucuronidase activity and decreased fecal concentrations of the pharmacologically active metabolite SN-38. It was associated with an improvement in diarrhea and not with hematologic toxicity, suggesting that bacterial β-glucuronidase plays a crucial role in irinotecan-induced diarrhea without affecting enterocyclic and systemic SN-38 levels (26).
Novel experimental substances may have relevance in this context. RDP58 is an anti-inflammatory agent that inhibits the production of tumor necrosis factor-α, interferon-γ, and interleukin-12. In animals, oral administration of RDP58 significantly decreased the incidence of diarrhea and improved the survival rates of mice treated with toxic doses of irinotecan or 5-FU. RDP58 may have clinical utility in cancer therapy by preventing treatment-associated GI toxicity and potentially increasing the effectiveness of chemotherapy (27).
Clonidine, an α2-agonist, has been reported to be useful in controlling diarrhea in patients with diabetes or in patients with chronic idiopathic secretory diarrhea who presumably present a denervation sympathetic supersensitivity. However, hypotension and sedation may limit the usefulness of clonidine in dehydrated patients or patients with advanced cancer (4).
Opioids have been traditionally used for their antidiarrheal properties owing to the widespread presence of opioid receptors at different peripheral sites, including smooth muscle, myenteric plexus, and spinal cord. It is well known that their activation increases ileocecal tone and decreases small intestine and colon peristalsis (increasing electrolyte and water absorption), also impairing the defecation reflex by inhibiting anorectal sphincteric relaxation and diminishing anorectal sensitivity to distention. As a consequence, the contact time between intestinal mucosa and luminal contents is enhanced by the reduction of colonic propulsive activity, resulting in greater fluid absorption (4). Antidiarrheal effects can be obtained by both oral and parenteral opioids. Among opioids, loperamide is more specific because of the prevalent peripheral effect due to the inability to cross the blood–brain barrier. Loperamide shows the highest antidiarrheal/analgesic ratio among the opioid-like agents and is proved to be the drug of choice because of its few adverse effects. The standard dose of loperamide is 4 mg followed by 2 mg after every unformed stool. The dosage is titrated against the effect and higher doses of 2 mg every 2 hours have been recommended (up to 12 mg per day or more) in conjunction with chemotherapeutic agents associated with a high incidence of diarrhea (28). Loperamide-simethicone combination was significantly more effective than the drugs taken alone in the treatment of acute diarrhea with gas-related abdominal discomfort (29). However, the risk of developing paralytic ileus in the presence of continuous secretion, should be considered as a life-threatening complication. Of interest, opioids may paradoxically cause diarrhea secondary to fecal impaction.
Data from several clinical trials suggest that octreotide may be useful in the symptomatic treatment of diarrhea refractory to other medications (28, 30). The mechanism by which octreotide produces these beneficial effects is probably multifactorial, as it suppresses the secretion of many of the gut peptides implicated in the control of secretory and motor activity, and inhibits exocrine secretions from the stomach, pancreas, and small intestine, also facilitating water and electrolyte absorption (31). Octreotide has been found to control diarrhea in several conditions, such as carcinoid tumors, vipoma, gastrinoma, small cell lung cancer, as well as acquired immunodeficiency syndrome–related diarrhea (32). However, hormonal responses to the somatostatin analog do not always parallel clinical responses, probably because of the effects of cosecreted peptides. A dose-response effect of octreotide has been demonstrated. Octreotide seems to be an effective agent in the management of chemotherapy-related diarrhea and refractory GVHD-associated diarrhea (33, 34). Doses of 0.3–1.2 mg a day subcutaneously are commonly effective.
Long-acting, biodegradable, microsphere formulation of octreotide for monthly subcutaneous administration (30 mg) has been evaluated for the prophylaxis of diarrhea, speeding the resolution of diarrhea and preventing further episodes during subsequent cycles of chemotherapy (64). A preventive strategy with octreotide LAR as prophylaxis has been proposed for patients with a prior cycle of chemotherapy complicated by persistent diarrhea (35).
Malabsorption
An ineffective absorption of breakdown products in the small intestine may occur either because a disorder interferes with the digestion of food or directly with the absorption of nutrients. The digestive and absorptive processes are inextricably linked. The series of events include the reduction of particle size, solubilization of hydrophobic lipids and enzymatic digestion of nutrients to small fragments, absorption of the products of digestion across the intestinal cells, and transport through lymphatics.
Physiopathology of Digestion
The pancreatic secretion of lipase, amylase, and proteases breaks down fat to monoglycerides and fatty acids, carbohydrates to mono- and disaccharides, and proteins to peptides and amino acids. Several processes have been recognized to facilitate the absorption of fat from the aqueous luminal environment. Triglycerides are emulsified together with phospholipids, bile salts, and mono- and diglycerides and dispersed into a variety of phases and particles. Lipid digestion begins in the mouth and in the stomach, active at a low pH, promoting emulsion stability and facilitating the action of pancreatic lipases. Gastric and pyloric motility further promotes emulsification of lipids. This effect is amplified by bile salts and biliary phospholipids, which also influence the absorption of cholesterol and sterol vitamins. Lipolysis to fatty acids and monoglycerides is mediated by pancreatic lipases (4). Protein digestion begins in the stomach. Acid denaturation leads to proteolysis, which is promoted by endopeptidases activated by an acid environment, cleaving the internal bonds of large
proteins to form nonabsorbable peptides. Pancreatic peptidases convert proteins and polypeptides into amino acids and oligopeptides. Hormonal and neural stimulation stimulates the release of proenzymes by the pancreas. Enteropeptidases and trypsin activate a cascade of events that promotes the activation of chymotrypsin, elastase, and carboxypeptidase A and B in the duodenum. Digestion of carbohydrates has been described in the section on Osmotic Diarrhea.
proteins to form nonabsorbable peptides. Pancreatic peptidases convert proteins and polypeptides into amino acids and oligopeptides. Hormonal and neural stimulation stimulates the release of proenzymes by the pancreas. Enteropeptidases and trypsin activate a cascade of events that promotes the activation of chymotrypsin, elastase, and carboxypeptidase A and B in the duodenum. Digestion of carbohydrates has been described in the section on Osmotic Diarrhea.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree