Lung cancer is the most frequent cause of mortality worldwide. According to recent estimates, 222,520 new cases of lung cancer (non–small cell and small cell combined) were diagnosed and 157,300 lung cancer-related deaths occurred in 2010 in the United States alone. The two major histologic types of lung cancer are small cell lung cancer and non–small cell lung cancer. The diagnosis and management of lung cancer requires a multidisciplinary approach.
Lung cancer is the most frequent cause of mortality worldwide. According to recent estimates, 222,520 new cases of lung cancer (non–small cell and small cell combined) were diagnosed and 157,300 lung cancer–related deaths occurred in 2010 in the United States alone. The two major histologic types of lung cancer are small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC). SCLC (oat cell) is the more aggressive cancer, with median survival of 2 to 4 months without treatment. However, it is usually responsive to systemic chemotherapy, with median survival rates of 18 to 36 months. Less prevalent than NSCLC, SCLC accounts for only 20% of all new lung cancers per year. In contrast, NSCLC accounts for 80% of all new lung cancers annually and is amenable to surgical excision in select patients. NSCLC includes three major subtypes: squamous cell carcinoma (30%–40%), adenocarcinoma (25%–30%), and large cell lung carcinoma (<10%). The diagnosis and management of lung cancer requires a multidisciplinary approach. The method of diagnosis of suspected lung cancer depends on the pathologic type, the size and location of the primary tumor, the presence of metastasis, and the overall clinical status of the patient.
Diagnosis
Patients with lung cancer usually present with an abnormal finding on a routine chest radiograph, such as a nodule or effusion. This study is generally followed by a contrast-enhanced chest CT scan, which should include examination of the liver and adrenal glands along with mediastinal lymph node stations. The overall accuracy of CT in mediastinal staging for lung cancer is only 0.75 to 0.80, with 20% to 40% false-negative and 18% to 23% false-positive results. Although integrated PET-CT scanning is more sensitive and specific, yielding up to 98% correct tumor staging compared with final histologic staging, it is not as widely available as CT.
A lymph node is considered malignant when its shortest axis is at least 10 mm. The four most common sites of lung cancer metastases (stage IV disease) are brain, bone, liver, and adrenal glands. Consequently, head CT, brain MRI, and bone scans are often part of the metastatic evaluation. In the absence of central nervous system or bony symptoms in patients presenting with small tumors, the incidence of a true-positive finding is less than 10%. If disease in these sites is excluded, the cancer is confined to the chest (stages I–IIIB).
Positron emission tomography (PET) relies on the uptake and concentration of 2,3-fluorodeoxyglucose (FDG) in lung cancer cells. It is more sensitive and specific (approximately 90% sensitive and specific) for mediastinal nodal metastases compared with CT. PET scan is also fairly sensitive in detecting distant metastatic disease throughout the body exclusive of the brain. PET has an overall sensitivity of 96% (range, 83%–100%), specificity of 79% (range, 52%–100%), and accuracy of 91% (range, 86%–100%). False-negative results can occur with lesions smaller than 1 cm because a critical mass of metabolically active malignant cells is required for PET diagnosis. Lowe and colleagues found a sensitivity of only 80% in lesions smaller than 1.5 cm, compared with 92% in larger lesions.
False-negatives can also occur in tumors with low metabolism, such as carcinoid tumors. False-positive FDG uptake, particularly in pulmonary nodules or mediastinal lymph nodes, is seen in inflammatory conditions such as bacterial pneumonia, pyogenic abscesses, and aspergillosis, and in granulomatous diseases, such as tuberculosis, sarcoidosis, histoplasmosis, Wegener granulomatosis, and coal miner’s lung. Guidelines for obtaining PET scan are shown in Box 1 .
FDG-PET imaging is recommended in patients with low to moderate pretest probability of malignancy (5%–60%) and an indeterminate solitary primary nodule (SPN) greater than 8 to 10 mm in diameter.
FDG-PET is not recommended to characterize the SPN in patients with high pretest probability of malignancy (>60%) or with SPNs less than 8 to 10 mm in diameter.
In patients with an indeterminate SPN greater than 8 to 10 mm in diameter who are candidates for curative treatment, observation with serial CT scans is an acceptable management strategy under the following circumstances:
- 1.
When the clinical probability of malignancy is very low (<5%)
- 2.
When clinical probability is low (<30%–40%) and the lesion is not hypermetabolic on FDG-PET or does not enhance greater than 15 Hounsfield units (HU) on dynamic contrast CT
- 3.
When needle biopsy is nondiagnostic and the lesion is not hypermetabolic on FDG-PET
- 4.
When a fully informed patient prefers this nonaggressive approach
In patients with an indeterminate SPN greater than 8 to 10 mm in diameter who are candidates for curative treatment, a transthoracic needle biopsy or bronchoscopy is appropriate under the following circumstances:
- 1.
When clinical pretest probability and findings on imaging tests are discordant; for example, when the pretest probability of malignancy is high and the lesion is not hypermetabolic on FDG-PET
- 2.
When a benign diagnosis that requires specific medical treatment is suspected
- 3.
When a fully informed patient desires proof of a malignancy before surgery, especially when the risk for surgical complications is high
In surgical candidates with an indeterminate SPN greater than 8 to 10 mm in diameter, surgical diagnosis is preferred under most circumstances, including the following:
- 1.
When the clinical probability of malignancy is moderate to high (>60%)
- 2.
When the nodule is hypermetabolic on FDG-PET imaging
- 3.
When a fully informed patient prefers to undergo a definitive diagnostic procedure
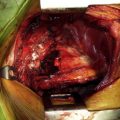
Approximately 70% of lung cancers are diagnosed and staged through small biopsies or cytology rather than using surgical resection specimens, with increasing numbers of transbronchial needle aspirations (TBNA), endobronchial ultrasound–guided needle aspirations (EBUS/TBNA), and esophageal ultrasound–guided needle aspirations being performed each year. Patients with suspected lung cancer who present with a pleural effusion should undergo thoracentesis first to differentiate between a malignant versus paramalignant effusion. When three separate pleural fluid specimens with malignant pleural disease are submitted to an experienced cytologist, a positive diagnosis can be expected in approximately 80% of patients, whereas percutaneous closed pleural biopsy is reported to be diagnostic for malignancy in approximately 50% of patients. Thoracoscopic biopsy of the pleura is safe and can provide a definitive diagnosis with a high degree of accuracy and minimal risk to the patient. In most instances it is performed as an outpatient procedure. The reported sensitivity rate for thoracoscopic biopsy ranges between 0.80 and 0.99, the specificity rate ranges between 0.93 and 1, and the negative predictive value ranges between 0.93 and 0.96. False-negative results are more common with mesothelioma than with primary lung carcinoma. In the case of a small (<3 cm) solitary peripheral lung lesion suspicious for lung cancer, the diagnostic dilemma generally centers on whether to obtain a biopsy specimen to confirm the diagnosis before surgical resection. In the presence of a high index of suspicion for lung cancer, an excisional wedge biopsy performed via video-assisted thoracic surgery has a much higher sensitivity than transthoracic needle aspiration (TTNA) and is the most definitive method of establishing a diagnosis. The diagnostic workup of lung cancer has evolved into a multidisciplinary approach. Patients suspected of having lung cancer require a histologic diagnosis and disease staging before appropriate treatment can be established. In addition, the development and clinical application of so-called targeted therapies require an increasing number of molecular investigations, such as gene amplification or mutation analysis, capable of scutinizing cancer at the nucleic acid level.
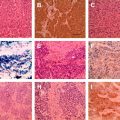
The histopathologic typing of lung tumors is performed according to the WHO criteria, but several specific antibodies are useful in establishing or eliminating diagnoses of primary lung cancers. A few examples of proved and reliable markers are available for a few common malignant tumor groups; large cell neuroendocrine carcinoma (chromogranin+, synaptophysin+, NCAM/CD56+, TTF-+/−, CK7+, CK high/lowMW+/−, AE1/AE3), SCLC (TTF-1+, CD56 or other neuroendocrine marker+, CK+, CK lowMW+, AE1/AE3, CK highMW-, LCA-, MIB-1/Ki-67 >50%), typical carcinoid (neuroendocrine marker+, TTF-1-, CK7, MIB1/Ki67<, <2 mitoses/2sqmm (10HPF), and atypical carcinoid (neuroendocrine marker+, TTF-1+/−, CK7+, MIB-1/Ki-67>, 2–10 mitoses/2sqmm and necroses). It also depends on the type of lung cancer, the size and location of the primary tumor, the presence of metastasis , the radiographic appearance of the lesion, and the overall clinical status of the patient .
SCLC
Bulky lymphadenopathy and direct mediastinal invasion are often associated with SCLC. A hilar mass is a particular characteristic of SCLC and is seen in approximately 78% of cases. SCLC may present with paraneoplastic syndromes, namely syndrome of inappropriate antidiuretic hormone, ectopic adrenocorticotropic hormone production, and Lambert-Eaton syndrome. SCLC can be diagnosed through sputum cytology, thoracentesis, fine-needle aspiration (FNA) of a supraclavicular node or metastatic site, and bronchoscopy with or without TBNA of mediastinal nodes or a submucosal process. When SCLC is diagnosed in a biopsy-proven specimen of the primary lesion, the next step is staging. One must determine whether the cancer is localized to the hemithorax or if extensive disease is present. Routine staging of SCLC includes a CT scan of the chest and abdomen or one of the chest with cuts through the entire liver and adrenal glands; a CT scan or MRI scan of the brain; and a bone scan.
NSCLC
In patients suspected of having NSCLC, the method for diagnosis is usually dictated by the presumed stage of the disease. Patients with metastatic NSCLC (stage IV disease) usually present with constitutional symptoms (eg, fatigue, weight loss), organ-specific symptoms (eg, bone pain, neurologic symptoms), or abnormal laboratory findings (eg, anemia, elevated alkaline phosphatase levels, elevated liver enzyme levels). In many of these patients, FNA or a needle biopsy of a site of metastasis represents the most efficient way to diagnose and confirm the stage of the disease. In some cases, however, the metastatic site may be technically difficult to biopsy. If metastatic disease can be predicted with a high degree of accuracy based on radiographic findings (eg, multiple brain, liver, bone lesions), using whatever method is easiest for the patient (eg, sputum cytology, bronchoscopy, TTNA) to diagnose the primary lung lesion may be more efficient. This decision must be made after weighing the technical considerations involved in each approach and the reliability of diagnosing an extrathoracic lesion as a site of metastasis based on radiographic appearances alone. A joint decision among the surgeon, radiologist, pulmonologist, and medical or radiation oncologist is the desirable approach.
NSCLC can present with extensive infiltration of the mediastinum, which is defined as a mass that infiltrates and encases the mediastinal structures, with no visible discrete mediastinal lymph nodes. In these patients, the method that has the most favorable risk/benefit ratio should be used for diagnosis. Bronchoscopy with TBNA for cytologic or histologic examination of mediastinal lymph nodes has been shown to be a safe procedure. Technical aspects that are frequently emphasized as important to a high success rate include accurate preparation of the specimen, rapid on-site evaluation by a cytopathologist, and use of the larger 19-gauge needles, which provide better tissue samples for histologic evaluation. The overall sensitivity of TBNA is 0.76 and the specificity is 0.96. , The negative predictive value of TBNA is not high enough (0.71) to obviate the need for further confirmation of negative results. Mediastinoscopy is warranted in patients with nondiagnostic results.
TTNA (CT-guided) of mediastinal masses can also be performed safely if needed. The role of TTNA in patients with extensive mediastinal disease (defined as such extensive mediastinal tumor growth that discrete lymph nodes can no longer be discerned) is usually to confirm the presence of SCLC or NSCLC; these patients are not surgical candidates because of the extent of mediastinal disease.
Finally, two major areas of interaction between specialties drive the need for a multidisciplinary approach to lung cancer diagnosis: staging and treatment. An international multidisciplinary classification was sponsored by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society. The terms bronchioloalveolar cell carcinoma (BAC) and mixed subtype adenocarcinoma were eliminated. The new classification of adenocarcinoma of the lung reflects the recent progress in molecular biology and oncology, such as the discovery of epidermal growth factor receptor (EGFR) mutation and its prediction of response to EGFR tyrosine kinase inhibitors (TKIs) in adenocarcinoma ; the requirement to exclude a squamous cell carcinoma diagnosis to determine eligibility for treatment with pemetrexed or bevacizumab ; the emergence of radiologic–pathologic correlations between ground-glass opacity and solid or mixed opacities seen on CT and BAC versus invasive lung cancer in patient prognosis; and improved preoperative assessment for choice of timing and type of surgical intervention.
For resection specimens, new concepts have been introduced, such as adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) for small solitary adenocarcinomas with either pure lepidic growth (AIS) or predominant lepidic growth with less than 5 mm invasion (MIA). These categories define patients who, if they undergo complete resection, will have 100% or near 100% disease-specific survival, respectively (further detail is provided in the article on pathology by Drs Fan and Schraeder elsewhere in this issue).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree