The Diagnostic Tools for Head and Neck Cancer
Giacomo Spinato1, 2, *, Paolo Boscolo Rizzo1, Marco Salvatore3, Simonetta Ausoni4, Samuele Frasconi1, Giuseppe Azzarello5, Carlo Cavaliere3, Liberatore Tramontano3, Maria Cristina Da Mosto1
Abstract
Diagnosis plays a key role in overall patient assessment and accurate staging of the malignancy. Diagnosis is the starting point to choose treatment strategies for the disease, as well as the basis upon which therapy success, prognosis and the patient’s quality of life will vary. Considering a high level of clinical suspicion of any mucosal alteration or laterocervical swelling is important until medical examinations provide evidence of the contrary. Diagnosis investigation continues with the search, among data collected on the patient’s history, of objective signs on which clinical suspicions will focus through further medical and instrumental examinations. Imaging techniques that can be used are ultrasound scan, computerised tomography, magnetic resonance and, in the most complex cases, positron emission computerised tomography. Ultrasonography is the most commonly used imaging technique for head and neck mass, especially for the assessment of lymph nodes, thyroid glands and salivary glands. Magnetic resonance is also considered an important examination in the diagnosis of head and neck tumours, especially for lesions involving the oral cavity, oropharynx, nasopharynx and larynx. Computerised tomography (CT) scan is especially useful when assessing the skull base involvement and the morphology of laryngeal malignancies, for example when the tumour extends over the perichondrium of cartilage structures, as well as when assessing function, i.e. evaluating the degree of chordal motility. In head and neck cancers (HNC), predictive factors namely biological characteristics that can be used to predict tumor response to a specific treatment, are currently remarkably lacking. Conversely, some bio-molecular parameters are recognized as prognostic factors of the disease, since they indicate tumor characteristics that inform about cancer outcome,
independently of treatment the patients will undergo. The most prominent prognostic factor for head and neck cancers is viral etiology, specifically HPV-mediated disease for oropharyngeal carcinomas and EBV-mediated disease for nasopharyngeal carcinoma.
* Corresponding author Giacomo Spinato: Department of Neurosciences, Section of Otolaryngology and Regional Centre for Head and Neck Cancer, University of Padova, Treviso, Italy; Tel: +39 0422322324; and Department of Surgery, Oncology and Gastro-enterology, Section of Oncology and Immunology, University of Padova, Treviso, Italy; E-mail: spin.giacomo@gmail.com
INTRODUCTION TO DIAGNOSTIC INVESTIGATIONS IN HEAD AND NECK CANCER
Head and neck cancers may, due to the onset and evolution of the disease and variability of its histology, present in either full-blown or subtler forms. Diagnosis plays a key role in overall patient assessment and accurate staging of the malignancy. Diagnosis is the starting point to choose treatment strategies for the disease, as well as the basis upon which therapy success, prognosis and the patient’s quality of life will vary. Approximately half of all head and neck cancers are diagnosed when the disease is at an advanced stage, most likely because the patient tends to see a specialist only when the disease is full-blown because earlier symptoms are often vague or neglectable [1, 2]. Diagnostic errors occur when, in light of one or more patient-reported symptoms, the physician diagnoses the wrong disease or when examinations and tests that are necessary for a correct diagnosis are not performed on the patient.
THE ANAMNESIS ROLE IN THE DIAGNOSIS
The first step to avoid errors in diagnosis is to proceed with an adequate investigation of the patient’s history, especially with the aim of building, from the very beginning, a trust relationship between the patient and physician that will last throughout the diagnosis and treatment pathway [3–5]. When investigating the patient’s history, in addition to examining the patient’s familiarity in relation to head and neck cancers, another important aspect is to investigate their lifestyle and habits to identify risk factors associated with this type of malignancies, especially tobacco and alcohol abuse and, more recently, promiscuous sexual habits [6–9].
In addition to lifestyle factors, investigating the patient’s profession is also fundamental at this stage, given the known correlation between prolonged exposure to toxic/irritating agents such as heavy metals or wood dust and the onset of upper aerodigestive tract tumours [10]. Agents that are irritating for the mucosa of the upper aerodigestive tracts produce a chronic mucosal phlogosis and can lead to metaplastic or dysplastic phenomena. Factors favouring the onset of chronic mucosal phlogosis in the upper aerodigestive tracts may include gastroesophageal reflux disease, a diet low in antioxidants – especially group A and E vitamins – and viral aetiologic agents, which should be investigated by testing malignant cells for EBV or HPV DNA in patients with nasopharyngeal or oropharyngeal carcinoma respectively [11, 12].
In the case of tumours of the thyroid and salivary glands, investigating any previous exposure to radiation therapy, especially during paediatric age, or high accidental exposure to radiation, as occurred in the past in the Chernobyl disaster, is essential.
Finally, the patient’s history investigation should focus on any patient comorbidities, organ failures, home-delivered therapies and allergies that may impact treatment decisions once the precise clinical staging is complete [13–15].
CLINICAL ASSESSMENT IN HEAD AND NECK CANCER
The analysis and accurate interpretation of reported symptoms and clinical signs is of extreme importance in the setting of head and neck cancers, because the signs and symptoms that may present based on the site of disease onset may vary and differ [16, 17].
Often, during the initial stage of the disease, symptoms might be silent or vague; often, head and neck cancer patients are socially marginalised or live in poor social conditions and thus do not see a specialist until symptoms are full-blown and the disease is already at an advanced stage [18]. Symptoms at onset may be local and most frequently include, based on their site, sore throat, the feeling of a lump, hoarseness, unilateral nasal bleeding and reported symptoms at onset such as ear pain and headache. The most typical symptoms of a malignant evolution in the upper aerodigestive tracts are odynophagia, increasingly severe dysphagia [19], “hot potato” voice, salivary pooling, dyspnea (initially upon exertion), cacosmia, bleeding in the mouth, weight loss. Symptom evolution and the relatively rapid increase in severity are an index for mass growth speed evaluation and, indirectly, tumour malignancy [20, 21].
A typical feature of tumours of the nasopharynx [22] is that the patient reports a generally unilateral feeling of ear fullness associated with the onset of otitis media with effusion after a generally short period of time – a typical onset manifestation in this tumour type. Regarding salivary glands, a setting in which tumours are more frequently benign, the generally slow growth of a lump, alternated with periods of regression (as typically occurs with cystadenoma lymphomatosum), is observed in the tail of the parotid gland near the angle of the mandible relative to the superficial parotid gland, or in the mouth and pharynx in some cases of deep parotid lobe cancer, where malignancies may resemble a peritonsillar abscess without signs of acute phlogosis. In case of submandibular and sublingual glands, a level I laterocervical swelling, or lump in the floor of the oral cavity, may be identified. This leads to associated symptoms such as limited tongue motility and trismus with infiltrations in the pterygoid muscles and masticator space, resulting in the patient having difficulties to eat [23, 24].
Regarding parotid masses, odynophagia, ulcers of the skin overlying the glands and even a reduction or deficit of hemiface motility due to more or less direct involvement of the facial nerve may subsequently appear. The degree of involvement of the facial nerve may be evaluated according to the House-Brackmann scale [25], which includes six different grades based on the residual function of mimic muscles or of the orbicularis oculi muscle.
The scheme in Table 1 summarises symptoms and tumour position based on the evolution of the malignancy [26–30].
The patient will present to a specialist evaluation in light of the described symptoms and clinical condition [32]. When investigating the patient’s history [33], the age may lead to consider certain tumour types. In clinical practice, suspicion of oropharyngeal or nasopharyngeal malignant [34], or more commonly benign tumours in isolated laterocervical swellings originating from the branchial arches or residue of the thyroglossal tract tends to be more frequent in younger than in older patients. Considering with a high level of clinical suspicion, any mucosal alteration or laterocervical swelling is anyway important until medical examinations provide evidence of the contrary. The patient’s history needs to take into account familiarity with malignant diseases, the type of dietary habits, daily or accidental exposure to risk factors in the workplace, any predisposing comorbidities, the patient’s lifestyle and sexual habits; all the information collected on such aspects shall then be considered when choosing the therapy.
A critical assessment of data collected on the patient’s history should be accompanied by a detailed analysis of the timing of the onset and evolution of symptoms and clinical signs mentioned in Table 1 [35–38].
| Anatomical Site | Signs and Symptoms at Onset | Advanced Signs and Symptoms |
|---|---|---|
| Nasopharynx | Posterior rhinorrhea, unilateral fullness, cough with haemoptysis | Headache, relapsing posterior epistaxis, cacosmia, unilateral ear pain, symptoms relating to the local invasion of adjacent tissues and skull base |
| Nasal fossae and paranasal sinuses | Anteroposterior rhinorrhea, cough with haemoptysis, epistaxis, or symptoms of obstructive sinusitis, including facial pain and congestion, sudden onset of nasal obstruction | Complete nasal obstruction, significant relapsing epistaxis, anosmia, septum or cutaneous ulcers, symptoms relating to the local invasion of adjacent tissues, swelling or a mass in the hard palate, upper gum, gingivobuccal sulcus, or cheek. Loose teeth, anesthesia of the skin of the cheek, upper lip, diplopia, and proptosis, trismus, anesthesia in the distribution of the fifth cranial nerve or paralysis of the third, fourth, or sixth cranial nerves |
| Oral cavity | Feeling of a lump in the mouth | Relapsing bleeding in the mouth, dysphagia, trismus, symptoms relating to the local invasion of adjacent tissues |
| Oropharynx | Feeling of a lump in the mouth | Relapsing bleeding in the mouth, dysphagia, swallowing dysfunction, nasal regurgitation and aspiration, unilateral ear pain, symptoms relating to the local invasion of adjacent tissues |
| Hypopharynx | Feeling of a lump in the mouth | Relapsing bleeding in the mouth, dysphagia, hoarseness, dyspnea, nasal regurgitation and aspiration. Symptoms relating to the local invasion of adjacent tissues |
| Supraglottic larynx | Hoarseness | Relapsing bleeding in the mouth, hoarseness, aphonia, dysphagia, dyspnea. Symptoms relating to the local invasion of adjacent tissues |
| Glottic larynx | Hoarseness, dyshonia | Relapsing bleeding in the mouth, hoarseness, aphonia, dysphagia, dyspnea. Symptoms relating to the local invasion of adjacent tissues |
| Hypoglottic larynx | Hoarseness | Relapsing bleeding in the mouth, hoarseness, aphonia, dysphagia, dyspnea. Symptoms relating to the local invasion of adjacent tissues |
| Cervical esophagus | Dysphagia | Relapsing bleeding in the mouth, hoarseness, dysphagia, dyspnea, nasal regurgitation and aspiration. Symptoms relating to the local invasion of adjacent tissues |
| Salivary glands | Asymptomatic mass | Dry mouth, V and VII cranial nerve deficit, trismus, dysphagia. Ear pain, symptoms relating to the local invasion of adjacent tissues |
CLINICAL EXAMINATION IN HEAD AND NECK CANCER
Diagnosis investigation continues with the search, among data collected on the patient’s history, of objective signs which clinical suspicions will focus on through further medical examinations. The first diagnostic approach consists of an accurate examination of the patient. A general inspection of the patient may confirm part of the information gained from data on the patient’s history, such as their nutritional state, smoke or alcohol abuse and willingness to collaborate; all such information is essential for subsequent therapy planning [39–41].
Ear, nose and throat examination should, first of all, include the observation of face, scalp and neck skin; furthermore, the presence of any suspicious swollen, ulcerating or dyschromic lesions or facial asymmetries should be assessed [42]. In case of clinical signs, further information may be suggested by an accurate superficial and deep palpation to assess lesion texture, fixity and tenderness, together with the presence of pus from superinfection or cutaneous ulceration. Palpation of parotid swelling and any way of the main salivary glands should always be performed using two hands and should be associated to the inspection of the type of sputum produced by salivary ducts in order to differentiate diagnosis from purely infectious diseases.
Neck palpation extends to the region behind the preauricular area and all the way to the supraclavear area, assessing evident laterocervical swellings and identifying any suspicious lymph node beds that deserve more in-depth diagnostic evaluation [43, 44]. Lymph node characteristics worthy of assessment by means of palpation include, in particular, texture (soft vs. hard), size, speed of evolution (higher in more aggressive forms), site (uni- vs. bilateral) and tenderness (which is typically absent in suspicious forms [45].
Lymph node beds should be examined at all levels whenever possible. The conventional and international American Head and Neck Society classification involves seven levels, each draining lymph from a specific cutaneous or mucosal anatomical site of the head and neck region; such levels are categorized as follows;
- Level IA (submental level), which includes the area extending from the hyoid bone inferiorly, the midline medially and the lateral margin of the anterior belly of the digastric muscle laterally;
- Level IB (submandibular level), which is comprised within the mandibular branch inferiorly, the anterior belly of the digastric muscle anteriorly and the stylohyoid muscle posteriorly;
- Level II (upper jugular chain level), which is delimited by the cranial base superiorly, the stylohyoid muscle medially, the hyoid bone inferiorly and the posterior margin of the sternocleidomastoid muscle posteriorly; based on the point of emergence of the accessory spinal nerve, a perpendicular line can be traced to divide sublevel II in bed IIA, located anteroinferior to it, and sublevel IIB, which includes lymph nodes located posterosuperior;
- Level III (middle internal jugular chain level), which is included between the hyoid bone superiorly, the belly of the sternocleidomastoid muscle externally and the inferior margin of the cricoid cartilage inferiorly;
- Level IV (deep jugular chain level), which is the most deeply located site in respect of the sternocleidomastoid muscle and is delimited by the posterior margin of its muscular belly posteriorly, by the inferior margin of the cricoid cartilage superiorly and by the upper margin of the superior clavicle inferiorly;
- Level V (posterior triangle level), which is comprised of the triangle delimited by the posterior margin of the sternocleidomastoid muscle anteriorly, by the lateral margin and belly of the trapezius posterolaterally; a line can be traced perpendicularly to the midline, following the accessory nerve passing through the cricoid cartilage to divide level V into sublevel VA, located superiorly to such line, and sublevel VB, located in the inferior section;
- Level VI (anterior jugular chain level), which is comprised within the hyoid bone superiorly, the carotid artery laterally and the superior margin of the sternal manubrium inferiorly;
- Level VII (superior mediastinal level), which includes the pretracheal, paratracheal and oesophageal folds extending from the level of the superior margin of the sternal manubrium to the innominate artery.
Lymph drainage occurring at each level is summarised below:
- Level IA (submental level) drains the anteroinferior portions of the mouth (lip, gums and tongue) and of the sublingual gland;
- Level IB (submandibular level) drains the mid-level of third middle of buccal structures (lip, palate, dental arch and tongue), of the lateral portion of the mental region and of the submandibular gland;
- Level II (superior jugular chain level) drains lymph coming from nasal cavities, structures belonging to the posterior portion of the oral cavity, the parotid gland, the supraglottic larynx and the pharynx;
- Level III (middle jugular chain level) drains the pharynx and larynx;
- Level IV (inferior jugular chain level) drains lymph circulating in the larynx, thyroid, hypopharynx and cervical oesophagus;
- Level V (posterior triangle level) drains the pharynx, parotid gland and, through the retro-auricular and occipital lymph node beds, the respective areas of the scalp;
- Level VI (anterior jugular chain level) collects lymph coming from the thyroid, muscles of the anterior cervical compartment, larynx and cervical oesophagus;
- Level VII (superior mediastinal level), which includes the pretracheal, paratracheal and oesophageal folds extending from the level of the superior margin of the sternal manubrium to the innominate artery.
During the clinical investigation of the mucosa, all upper aerodigestive tract regions should be inspected with the help of both the simplest tools, such as headlight, cold spatula and nasal speculum and more sophisticated equipment such as white or filtered light optics to identify any suspicious, ulcerating, dyschromic, hyperplastic or neoangiogenic lesions [46, 47]. Ear examination using an otoscope, microscope or endoscope is essential for differential diagnosis of reported ear pain versus otodynia, as well as in the evaluation of the skin of the outer ear.
The accurate examination of the oral cavity, which is often underestimated, should involve various areas. To begin with, the condition of the patient’s dentition and gum mucosa of both dental arches may provide a significant amount of information on the patient’s overall conditions and personal care and hygiene, their lifestyle habits, any mucosal lesions due to poor oral hygiene, chronic trauma caused by sharp teeth or inadequate removable prostheses; moreover, a class II malocclusion and significant development of superior incisors may be a criterion for exclusion from transoral surgery. The accurate description of the dental state may furthermore prevent subsequent medical-legal post-intubation litigations or surgery in case of trauma or chipped teeth. Tongue examination considers aspect, hydration and any morphological or motility asymmetries of the mucosa. The examination of the floor of the oral cavity also includes the assessment of submandibular and sublingual gland excretory ducts. When suspicious lesions are recognized in any site of the oral cavity, accurate palpation follows in order to gain further information on its size and any infiltration of the malignancy. Different from the oral cavity, the oropharynx features sites that cannot be viewed directly, such as the tonsillar crypts or basis of the tongue so, in case of suspicion following an inspection or optic fibre pharyngoscopy, seeking clinical confirmation through deep palpation remains fundamental.
Portions of the head and neck area excluded from direct inspection and anterior rhinoscopy, i.e. nasopharynx [48, 49], nasal fossae posteriorly, part of the oropharynx and the area of the hypopharynx and larynx, require second-level tools that enable a full exploration. Based on convenience and clinical experience, either rigid or flexible endoscopic tools can be used. Nowadays, assessment of upper aerodigestive tracts by means of indirect pharyngolaryngoscopy specula is no longer sustainable in oncologic clinical evaluation, as the latter are not precise enough in terms of image resolution and are strictly operator-dependant. In rhinoscopy and endoscopy, suspicious – especially unilateral, ulcerating or bleeding – lesions should be sought.
Regarding the nasopharynx from choanae to the soft palate, obtaining evidence on the palatovelar function in swallowing and phonation, as well as on the patency of tubal ostia is important. Assessment of the hypopharynx and larynx, as well as the oropharynx as a whole, is feasible by means of rigid, angulated optic fibre video laryngoscopy, although the latter is not always accepted by patients with marked emetic reflex in spite of locally applied anaesthesia, which often facilitates evaluation. In the latter cases, a fibroscopy is performed using a flexible endoscope that bypasses the palatoglossal arch causing the emetic reflex.
The hypopharynx is a particular and anatomically complex structure consisting of portions that are clearly visible at endoscopy and expandable regions, such as the pyriform sinuses and retrocricoid region. An important, indirect suspicious clinical clue regarding expandable regions involves salivary pooling or lumen asymmetry when a clear investigation of the mucosa is not possible. The larynx includes subsites that can be more or less visible with laryngoscopy. While lesions of the glottic plane are easily recognisable even at an initial stage because of the early presence of hoarseness, other lesions, such as those in the hypoglottic cone and laryngeal ventricle, have a subtler clinical, symptomatic and laryngoscopic manifestation. Alterations in the motility of the hemilarynx can also be linked to conditions relating to mediastinal diseases. Information that is particularly important to collect when describing the larynx includes the patency of pyriform sinuses – areas from where squamous cell cancers frequently originate – as well as the aspect of motility of the cricoarytenoid units, which typically appear altered in the presence of suspected infiltrating lesions.
While high definition (HD) light optic fibre endoscopies enable a macroscopic view of mucosas, narrow band imaging (NBI) and autofluorescence technologies [50–53] allow, by means of specific filters, to distinguish the pathological lesion, characterised by its typical anarchic hypervascularisation, from the rest of the healthy mucosa, leading to early lesion diagnosis and directing further biopsy investigations. Beyond what is made possible by clinical examinations pertaining to the ENT specialist’s competence, using diagnostic radiography is anyway essential to continue the diagnosing pathway – thus the already mentioned importance of a multidisciplinary approach in the treatment of an oncologic disease. As a matter of fact, diagnostic radiography images enable specialists to stage the disease by knowing its actual local and, if applicable, distant depth extension.
IMAGING TOOLS IN HEAD AND NECK
Cross-sectional imaging techniques are ultrasound (US) scan with or without fine-needle aspiration cytology (FNAC), contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI) with or without diffusion-weighted (DW) sequences (DW MRI), positron emission tomography (PET)/CT or a combination of these techniques [54].
Each modality has its own strengths and drawbacks and findings provided by the different imaging modalities are usually complementary. The imaging role encompasses for the detection/exclusion of lesion, its characterization in terms of size, extent, regional lymph nodal involvement, perineural or perivascular spread, bone infiltration or long-ranging metastasis. Advanced imaging is also used to address biopsies, to decide and plan tailored treatments, and to assess/follow-up treatment response in head and neck tumors.
According to local (Tumor size and surrounding diffusion – T), regional (involvement of lymph nodes – N) and distant (presence/absence of metastases – M), the union for international cancer control (UICC) has classified head and neck tumors into different stages (TNM classification), determining a specific treatment (surgery and/or chemotherapy and/or radiotherapy) and prognosis. While different histological subtypes and anatomical subsites have different T staging [55], the N parameter is related to the site, number and laterality of the lymph nodes involved by the primary tumour site.
N staging for most tumours (except the nasopharynx and thyroid)
Nx means that the regional lymph nodes cannot be assessed
N0 no node involvement
N1 single ipsilateral lymph node 3 cm or less in greatest diameter
N2a single ipsilateral lymph node between 3 and 6 cm
N2b multiple ipsilateral lymph nodes less than 6 cm
N2c contralateral or bilateral lymph nodes less than 6 cm
N3 any lymph node larger than 6 cm.
N staging of nasopharyngeal tumours (midline nodes are considered ipsilateral)
N0 no node involvement
N1 ipsilateral single lymph node above the supraclavicular fossa, and/or unilateral or bilateral retropharyngeal lymph node(s), 3 cm or less in greatest dimension
N2a ipsilateral single lymph node between 3 to 6 cm
N2b multiple ipsilateral lymph nodes less than 6 cm
N2c contralateral or bilateral lymph node(s) 6 cm in greatest diameter above the supraclavicular fossa
N3a lymph node or nodes larger than 6 cm, unilateral or bilateral
N3b extension to the supraclavicular fossa
N staging for thyroid cancer
N1a level VI (pretracheal, paratracheal, prelaryngeal/Delphian lymph nodes)
N1b unilateral, bilateral or contralateral cervical (level I-V), retropharyngeal or superior mediastinal (level VII) nodes.
Distant metastases represent another crucial prognostic factor conditioning the therapeutical approach, referring to M0 for no distant metastasis and M1 for diffusion to distant organs beyond the regional lymph nodes. Al already mentioned, each imaging modality has intrinsic advantages and weakness in a so complex district as the head and neck region, differently contributing to each of the T, N, and M parameter.
As for the primary tumor assessment (T parameter), MRI, and CT differently contribute to the diagnostic phase, according to the subset and the clinical issue, while US has a limited role and it is performed mainly to evaluate salivary gland tumors, drive biopsies or to characterize/monitoring regional lymph nodal involvement [56]. Thanks to the colour Doppler technique, it enables to assess the way vascularisation behaves in the area, which can be peripheral or, in suspicious cases, intralesional with an increased flow. PET/CT, instead, is commonly prescribed to assess regional nodal infiltration and/or distant metastases in advanced stage head and neck tumors, and it is commonly performed with 18 fluoro-deoxyglucose tracers, which is preferentially transported and trapped into hypermetabolic cancerous or inflamed tissues.
MRI represents the imaging gold standard to characterize nasopharyngeal malignancy because of its superior contrast resolution, mainly for the assessment of adjacent soft tissue invasion (e.g., the pharyngobasilar fascia, sinus of Morgagni, skull base, intracranial involvement, and perineural spread). CT, instead, provides meaningful information regarding possible bony invasion, mainly for sinonasal pathology, and for the planning of open or endoscopic sinus surgery.
Regarding the carcinomas of the oral cavity and oropharynx, the choice of the cross-sectional imaging technique is often tricky, for example, due to dental hardware artifacts, mainly for CT scan, or movement artifacts due to patient swallowing and breathing, mainly for MR acquisition. While according to clinical issue, CT often represents the first step to define possible mandible and maxilla invasion, MR is more informative to establish bone marrow invasion and perineural spread, or to characterize small soft tissue tumors, such as primary tongue tumor’s submucosal extension [57–59].
Finally, considering the primary laryngeal or hypopharyngeal malignancies, CT is generally preferred to assess cartilaginous erosion, almost for the MR longer acquisition time that can be affected by swallowing artifacts [60]. Head and neck oncological lesions can appear as an asymmetry of the aerial lumen, or an asymmetrical thickening of the mucosa, or a soft tissue density mass on CT, that generally show a T2-weighted hyperintense signal and a T1-weighted hypo-isointensity on MR sequences, compared to healthy muscle [61]. If absent procedural contraindications like severe renal failure or previous anaphylactic reaction to contrast media, all the histological subtypes can enhance after contrasted medium injection, according to their vascularity. The more recent introduction of diffusion-weighted sequences (DW-MRI) can help to better identify hypercellular tumors, and mainly to distinguish between a disease relapse from phlogosis/scars caused by post-treatment alterations during the follow-up.
As for bony invasion assessment, imaging findings are often represented by a discontinuity of the normal hyper-attenuating cortical on CT or hypointense signal on MR, that can be paralleled by a medullary alteration, better detected by short tau inversion-recovery (STIR) sequence on MRI or spectral acquisitions on modern CT scan [61]. Regarding perineural diffusion, while CT shows only indirect signs as foramina enlargement or surrounding soft-tissue inhomogeneity, MRI can highlight a thickening of the nerve with a clearer hyperintensity following contrast medium injection [61].
Finally, considering the complementarity of information provided by PET/CT and MRI, several technological efforts have been done to combine these two modalities in a simultaneous PET/MR scanner. This need arises from the evidences of a relatively poor soft-tissue contrast especially when using low-dose PET/CT acquisition protocols or when intravenous contrast material is not injected, and from the additional CT radiation dose administered to the patient, that can have detrimental effects mainly in pediatric patients and in strict longitudinal follow-ups [62].
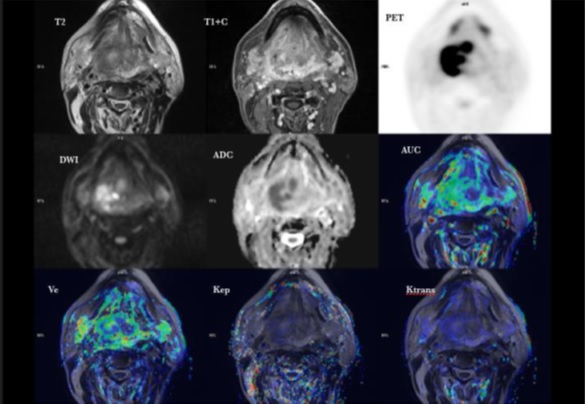
Despite the initial euphoria generated by the implementation of PET/MRI scanners for clinical use (Fig. 1), scientific data evaluating the clinical usefulness of hybrid PET/MRI systems remain at an early stage, and only very few studies have so far addressed the clinical workflow, feasibility and optimized imaging protocols in the head and neck [63–69].
Fig. (1))
PET/MRI of tongue tumour.
As for N parameter, detection of nodal metastases occurs in about 50% of patients at diagnosis, deeply affecting prognosis and survival rates in head and neck cancers [70]. Although the need for an accurate imaging method to assess lymph nodes is paramount, both CT and MRI, commonly used to characterize nodal involvement, missed at least an half of occult metastases [71]. This finding is due to the evidence that all currently available diagnostic imaging methods for nodal involvement assessment, rely on size (transversal short axis diameter of more than 1.5 cm for Level II nodes, and more than 1 cm for all other nodes) and morphologic criteria (rounded shape and loss of fatty hilum) [72–75] and that the identification of lymph node necrosis, the most predictive finding, has a limited role in smaller nodes (< 3cm) [76, 77]. Imaging signs that suggest an extracapsular nodal spread include capsular enhancement and irregularity, and infiltration of adjacent fat or muscle planes [61, 78–82]. The extracapsular spread is a crucial prognostic factor, considered the increased locoregional failure if treatment consists of only surgery.
Although, hybrid PET/MRI has demonstrated higher accuracy in the assessment of primary tumor (T-staging), no significant added value has been shown for lymph nodes involvement (N-staging) and limited improvement for metastasis detection (M-staging) when compared to PET/CT scan [83, 84]. As for imaging follow-up in head and neck cancer, an effort has been made by the ACR to standardize imaging surveillance and reporting with the introduction in August 2016 of the Neck Imaging Reporting and Data Systems (NI-RADS) [85]. Originally developed for CECT [86], NI-RADS template can be adapted to other cross-sectional imaging techniques [87] categorizing the disease’s status from 0 to 4, according to imaging suspicion (0 – incomplete, 1- negative, 2- low suspicion, 3- high suspicion, and 4- definite recurrence). Nevertheless, it has to be taken into account that imaging pattern varies accordingly to the type of treatment received, including edema, inflammation, fibrosis, and others that can render tricky differentiate between tumor recurrence and post-treatment alterations in the early stages [88].
More recently, radiomics technique has been introduced [89–91], as an high-throughput extraction approach of quantitative imaging features, able to (I) non-invasively characterize the overall tumor accounting for heterogeneity; (II) produce prognostic and/or predictive biomarker value derived from routine, standard of care imaging data as-is; and (III) allow for a fast, low-cost, and repeatable means for longitudinal monitoring [92–94]. This kind of approach can be applied to all cross-sectional imaging techniques to non-invasively disentangle among the heterogeneous composition of head and neck cancers toward a personalized patient’s treatment. Several promising applications of radiomics framework have been applied to classify and segment head and neck cancers. For instance, different studies have classified head and neck tumors by human papillomavirus (HPV) status with textural analysis [95–98], or segmented in an automatic manner healthy by cancerous tissue [99–101].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree