Fig. 7.1
Contract-enhanced CT demonstrates vessel erosion in a consolidating infiltrate of the left lung. An apposition thrombus can be depicted in the pulmonary artery of the left lower lobe of this 45-year-old female who underwent unrelated PBST 6 months before due to AML. She died 2 days later from brain infarction
7.1.3 CT
The advantage of HRCT in comparison to CXR for the early detection of pneumonia was demonstrated in febrile neutropenic patients not responding to empirical antibiotic therapy [21]. In approximately 60 % of the patients with a normal CXR, HRCT showed pulmonary infiltrates (Fig. 7.2). In only 10 % of patients with a normal chest x-ray and a normal HRCT, pneumonia occurred during follow-up [21]. Exclusion of pneumonia is another clinically relevant information. Thus, CT yields very useful results with good sensitivity (87 %) and negative predictive value (88 %). The early use of HRCT achieves a gain of approximately 5 days during which pneumonia may be excluded [17]. In clinical practice, this may be very helpful for the management of immunocompromised hosts at high risk of life-threatening pulmonary infection [5] (Fig. 7.2).
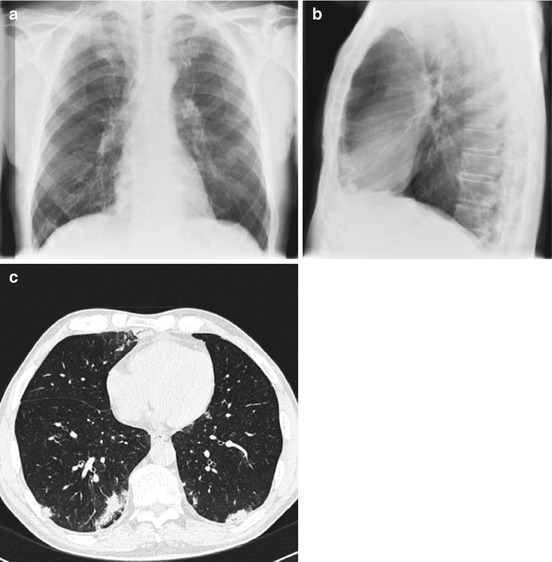

Fig. 7.2
Neutropenic febrile patient receiving broad-spectrum antibiotic therapy. CXR was normal at day 3 of fever (a, b). HRCT performed the same day demonstrates bilateral infiltrates, which were hidden behind the heart in posterior-anterior and the spine in lateral projection (c)
7.1.4 Magnetic-Resonance Tomography (MRI)
MRI has been evaluated for the investigation of pulmonary disease since it has a known benefit in lesion characterization [29, 30]. Comparing CT to MRI on an intraindividual basis, MRI reveals comparable clinical results (sensitivity 95 %, specificity 88 %, positive predictive value 95 %, negative predictive value 88 %) [53]. Besides the lack of radiation, there is no clear advantage of MRI in the early detection of pneumonia (Fig. 7.3). In advanced stages, CT and MRI are comparable in the visualization of infiltrates [30]. CT is widely available, easier, and faster to perform as well as less susceptible to breathing artifacts. MRI is superior to CT in the detection of abscesses due to a clearer detection of central necrosis in T2-weighted images and rim enhancement after contrast application in T1-weighted images [29]. However, this fact has limited clinical impact and duration of MRI, and required compliance is substantially higher compared to CT. MR has problems to detect small lesions and those which are adjacent to the left ventricle due to the cardiac motion [53].
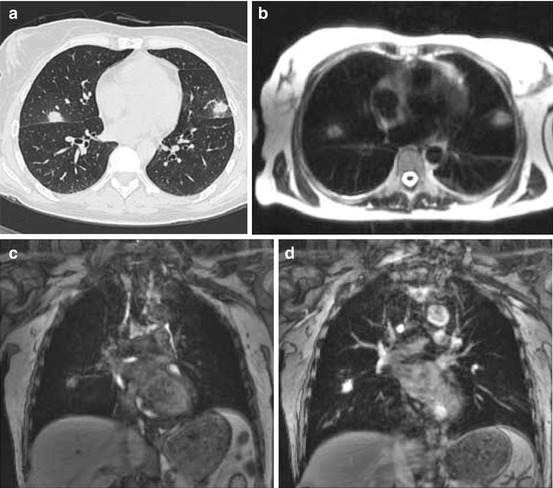

Fig. 7.3
Fungal pneumonia in HRCT (a), T2-weighted (b), non-enhanced T1-weighted GE MRI (c) and after Gd application performed the same day (d). Lesion contrast is similar in CT and contrast-enhanced MRI
7.1.5 Recommendation for Clinical Practice
In contrast to systemic infections, identification of the underlying organism in pneumonia is more difficult and complex. Attempts to reinforce pathogen identification did not improve the clinical outcome significantly [9]. Therefore, a calculated (preemptive) decision on antimicrobial therapy in febrile immunosuppressed patients based on imaging studies is widely used.
The use of CT is recommended for the early detection of pneumonia [9]. It may serve for indication and localization of invasive diagnostic procedures such as bronchoscopy and bronchoalveolar lavage or CT-guided biopsy. On the other hand, the exclusion of pneumonia can be obtained with a higher reliability as compared to conventional CXR. The sequential cascade as shown in Fig. 7.4 can be modified if the local institutional CT capacity allows for the skipping of CXR.
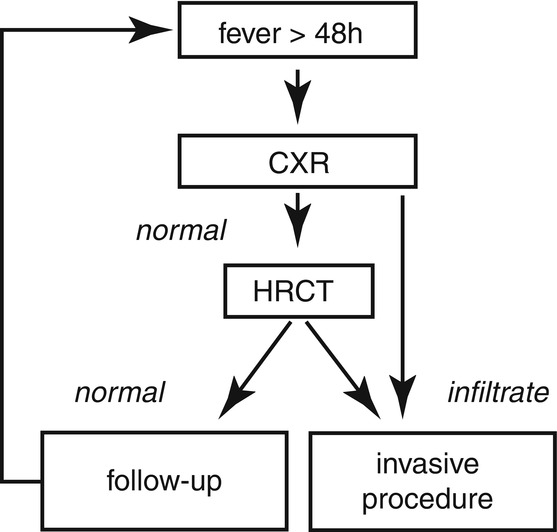

Fig. 7.4
Recommendations of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) [13]. Since the initial CXR is of limited use, this diagnostic step is more and more omitted and CT is performed primarily
7.2 Monitoring of Lung Infiltrates
An increasing size of pulmonary infiltrates during hematopoietic recovery has been well described by [31]. Caillot et al. evaluated HRCT in neutropenic patients with proven pulmonary aspergillosis at weekly intervals [31] and documented the time points of different radiological patterns and evaluated the size of infiltrates and documented the time points of different radiological patterns and evaluated the size of infiltrates. They frequently found a “halo sign” (Fig. 7.5) on the first CT scans and reported a low sensitivity of this pattern (68 %), which was no longer visible on follow-up scans. In contrast, the more specific “air-crescent sign” (Fig. 7.7) emerged frequently during follow-up (up to 63 %). The size of infiltrates increased by fourfold under successful antifungal treatment due to hematopoietic reconstitution. In this study, pneumonia was first detected on day 19 of neutropenia. Enlargement of infiltrates is probably caused by the invasion of newly generated neutrophil granulocytes at the beginning of bone marrow recovery. In critically ill patients, leukocyte invasion has been described as a risk factor for the development of acute respiratory distress syndrome (ARDS) [14] (Fig. 7.6 and 7.7).
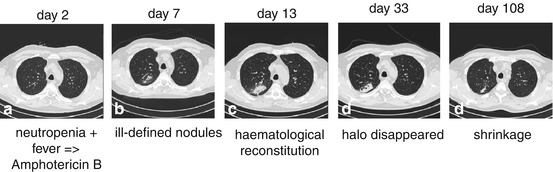
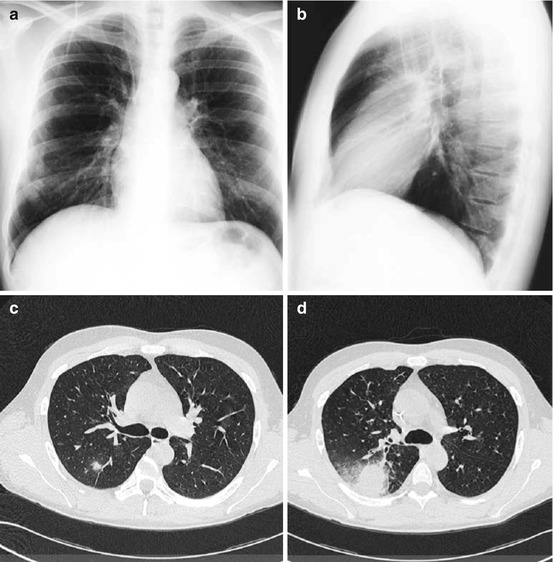
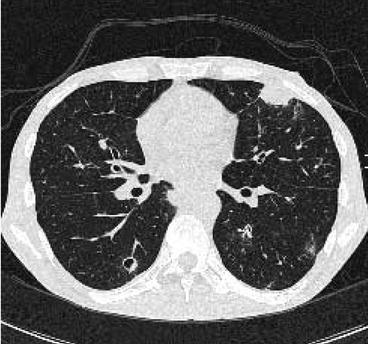

Fig. 7.5
Neutropenic febrile patient who underwent autologous peripheral blood stem cell transplantation. On day 2 after posttransplant, empirical mold-active antifungal treatment was started for neutropenic fever. Ill-defined pulmonary nodules were detected on day 7. Due to marrow recovery on day 13, the volume of lung infiltrates reached its maximum. Under continued antifungal treatment, the halo slowly disappeared and a central cavitation developed (day 33). The lesions shrinked significantly until day 108

Fig. 7.6
The small ill-defined nodule in the right upper lobe (c) of this 34-year-old neutropenic AML patient was even retrospectively not visible on conventional chest x-ray done at the same day (a, b). Amphotericin B treatment was started due to suspicion of fungal pneumonia; however, the nodule size increased during hematopoietic reconstitution 2 weeks later (d). In preparation of bone marrow transplantation, the lesion was surgically resected and Aspergillus pneumonia was verified

Fig. 7.7
Non-fungal lung infiltrate: ill-defined nodules with cavitation on CT scans done due to repeated febrile episodes. They appeared like fungal pneumonia and were not visible on baseline CT. After removal of a central venous port system, both the pulmonary lesions as well as the febrile episodes, disappeared
7.3 Characterization of Pneumonia
Radiologists’ dream is to be capable to identify the underlying microorganism in pneumonia of immunocompromised hosts with a sufficient specificity. In some cases, imaging can provide very fast useful hints, but no verification. The quality of these clues depends on the cooperation between clinicians and radiologists and on the radiologists’ experience with these complications. This requires an informational exchange concerning relevant individual patient data like severe neutropenia or allogeneic stem cell transplantation. For example, information on reactivation of cytomegalovirus (CMV) in a patient with graft-versus-host disease is very helpful for the correct interpretation of pulmonary HRCT findings. Also the chemotherapy or total body irradiation applied for conditioning before aSCT may be relevant for differential diagnostic considerations in patients who might present with clinically similar signs and symptoms [6, 7, 32, 33]. Some of the most useful clues are listed in Table 7.1.
Table 7.1
Clinical and radiological appearance for various infectious and noninfections lung abnormalities in neutropenic hosts and after allogeneic stem cell transplantation
Diagnosis | Clinical setting | Radiological appearance |
|---|---|---|
Infection bacterial | Early phase neutropenia | Consolidation, bronchopneumonia positive pneumobronchogram, GGO |
Fungal | Long-term neutropenia (>10 days) | Halo = ill-defined nodules (early phase) |
Consolidations negative pneumobronchogram | ||
Air-crescent sign/cavitation (late phase) | ||
Pneumocystis | Allogeneic transplantation | GGO, spared-out subpleural space |
Intralobular septae (late phase) | ||
Tuberculosis | Each | Small ill-defined nodules/cavitations, tree in bud, homogeneous consolidation |
Viral | Transplant history in graft or host | GGO – mosaic pattern |
Graft versus host | Allogeneic transplantation | GGO – mosaic pattern |
Intralobular septae become visible | ||
Tree in bud | ||
Air-trapping (expiratory CT) | ||
Radiation toxicity | Total body irradiation | GGO – paramediastinal distribution, also after TBI |
Intralobular septae become visible | ||
Drug toxicity | Bleomycin, methotrexate, high-dose cytarabine, carmustine, etc. | GGO – mosaic pattern |
Intralobular septae become visible | ||
Peripheral consolidations of secondary lobule | ||
Traction bronchiectasis | ||
Pulmonary congestion | Extensive hydration, renal impairment, hypoproteinosis | GGO |
Interlobular septae become visible | ||
Leukemic infiltration | Pulmonary involvement | Thickening bronchovascular bundles thickening |
Interlobular septae become visible | ||
GGO | ||
Pulmonary hemorrhage | Thrombocytopenia, post-interventional, hemoptysis | GGO – sedimentation phenomenon |
7.3.1 Bacterial Pneumonia
Bacteria are causing approximately 90 % of infections during the early phase of neutropenia [8]. The radiological appearance of bacterial pneumonia includes consolidation, especially bronchopneumonia and positive pneumobronchogram (Fig. 7.2) [33, 34]. In contrast to immunocompetent patients, ground-glass opacification is found more often and remains nonspecific.
7.3.2 Fungal Pneumonia
Severe neutropenia lasting for more than 10–14 days is associated with an increasing risk of invasive fungal infection [3], with Aspergillus species being the primary pathogen, while Candida species very rarely cause primary pneumonia (Fig. 7.8) [5]. Typical radiological findings of fungal and non-fungal pneumonia as well as of infiltrates from noninfectious diseases have been reviewed in detail [35]. The typical appearance of pulmonary infiltrates from fungal origin are as follows:
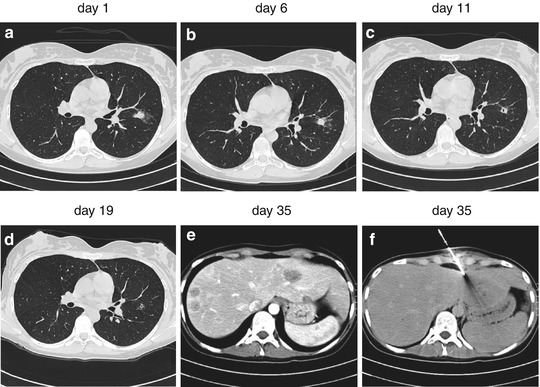

Fig. 7.8
Bilateral ill-defined nodules prompted the suspect of a fungal infection which was treated accordingly. Candida spp. were isolated from blood culture. Due to increasing liver enzymes, contrast-enhanced CT scan of the liver was ordered. Biopsy from the detected lesions confirmed Candida spp.
For use in the context of clinical and epidemiological research in neutropenic patients, standards for the interpretation of radiological findings in invasive fungal infections have been elaborated [10, 51]; newly emerged “typical” CT patterns (dense, well-circumscribed lesions with or without a halo sign, air-crescent sign) are classified as a clinical criterion for fungal pneumonia Figs. 7.5 and 7.8. The halo sign, first described in 1984 [49, 50], is nonspecific [33] and not a necessary part of the updated definitions [51]. A nonspecific infiltrate, rated as a minor criterion in the first version [10], was abandoned in the update [51]. In a later workup of a pharmaceutical trial investigating response rates to antifungal treatment, the evidence of the halo sign was associated with an improved response rate (52 % vs. 29 %; p < 0.001), as well as a higher 3-month survival rate (71 % vs. 53 %; p < 0.01) [52]. This large (n = 235) antemortem trial suffers, however, from systemic limitations like investigation of halo which was part of inclusion criteria and technical insufficiencies like usage of thick-section CT instead of appropriate thin-section CT and evaluation of hardcopies instead of monitor reading [52]. Histopathological workup of lung biopsies verified fungal pneumonia in 56 % of cases in another study [37]. Relevant differential diagnoses for the halo sign, such as cryptogenic organizing pneumonia (COP, formerly known as bronchiolitis obliterans organizing pneumonia, BOOP), pulmonary hemorrhage, pulmonary manifestation of the underlying malignancy, lung cancer, and non-fungal infections (CMV, tuberculosis, abscesses (Fig. 7.7), etc.) or Candida (Fig. 7.8), have to be considered [37].
Thus, diagnostic clarification will frequently be necessary, particularly when antifungal treatment is not successful.
Air-crescent sign and cavitation occur with hematopoietic reconstitution during the late phase of fungal infection (Fig. 7.8) [36]. Both radiological signs are known to be associated with a favorable prognosis. However, the specificities of these findings are limited, and relevant differential diagnoses have to be considered (Fig. 7.7) [37]. There are other useful patterns for the identification of fungal pneumonia, e.g., distribution along the bronchovascular bundle resulting in the feeding vessel sign with an angiotrophic location.
The ongoing development of antifungal therapy may have an important impact on the radiological appearance of fungal pneumonia. Thus, in the near future radiologists will not only be confronted with the question for “breakthrough fungal pneumonia,” but also for fungal pneumonia caused by non-Aspergillus pathogens.
7.3.3 Pneumocystis jiroveci Pneumonia (PcP)
Pneumocystis jiroveci pneumonia (PcP) [38] is a typical finding in hematological patients affected by severe cellular (T-cell) immunosuppression and those with graft-versus-host disease after aSCT, if they are not protected by effective chemoprophylaxis [8]. Despite standard trimethoprim-sulfamethoxazole prophylaxis, 8 % of the patients develop PcP, while among patients without prophylaxis, the incidence may reach 29 % [8]. Up to 15 % of these patients will have a fatal outcome [8].
CT provides a valuable characterization for PcP [6, 7, 18, 33] and is a reliable method for discriminating it from other infectious processes [33, 39]. A combination of ground-glass opacities and intralobular septae sparing out the subpleural space (i.e., perihilar distribution) is very typical for PcP (Fig. 7.9) [33, 39, 40].
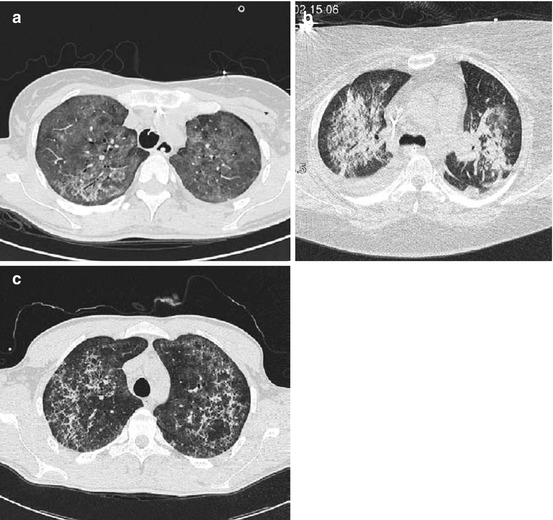

Fig. 7.9
Bilateral pneumonia caused by Pneumocystis jiroveci at different stages of immunosuppression. The subpleural space is typically spared out. Diffuse ground-glass opacification appears typically in the early phase of infection (a), while consolidations appear during a fulminant course (b). The predominance of intralobular linear patterns becomes visible during a later stage of PcP and under antimicrobial treatment (c)
7.3.4 Lung Tuberculosis
Tuberculosis (TB) has to be considered as a rare but relevant differential diagnosis. In immunocompromised hosts, TB appears different compared to immunocompetent hosts (e.g., gangliopulmonary (primary) forms) [41]. More widespread lymphogenic and hematogenous dissemination can occur, and therefore, the clinical course might be fulminant [41, 42]. On the other hand, TB might mimic or come along with other infections like pulmonary aspergillosis or systemic candidiasis [42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree