Chapter 1 Stephen F. Hawkins1 and Quentin A. Hill2 1 Department of Haematology, Royal Liverpool University Hospital, Liverpool, UK 2 Department of Haematology, Leeds Teaching Hospitals NHS Trust, Leeds, UK Anaemia was defined by the World Health Organization as a haemoglobin (Hb) concentration less than 120 g/L (Hb < 36%) in females and less than 130 g/L (Hb < 39%) in males, but the lower level of the reference range for Hb may vary between laboratories. It is common in critically ill patients, occurring in up to 80% of those in intensive care units (ICUs) [1] with 50–70% having a Hb less than 90 g/L during their admission. By reducing oxygen delivery to the tissues, this may be tolerated poorly in those with cardiorespiratory compromise. In critical care, anaemia is commonly due to multiple factors such as inflammation, blood loss, renal impairment and nutritional deficiencies [2, 3] (see Table 17.1), but it is important to consider treatable causes and identify when more detailed investigation is needed. The role of transfusional support is considered in Chapter 17. Tissue hypoxia exerts physiological control of Hb by triggering the release of erythropoietin (EPO) by the kidneys, which stimulates bone marrow (BM) erythropoiesis. Hb will increase so long as there are no underlying BM disorders (e.g. myelodysplasia) and there are adequate supplies of iron, vitamin B12 and folic acid. When a rapid marrow response occurs (e.g. following haemorrhage or replacement of a deficient vitamin), reticulocytes (young erythrocytes) enter the blood in large numbers and can be identified on the blood film (polychromasia) or by an elevated reticulocyte count. The normal lifespan for erythrocytes is 120 days, before being removed by the reticuloendothelial system (predominantly in the spleen and liver), but their lifespan may be shortened by inflammation, haemorrhage or haemolysis. In the history, note pre-existing co-morbidities including renal and cardiac impairment. Also note medications, diet and symptoms suggestive of blood loss. Examine for jaundice, lymphadenopathy or organomegaly. If the cause of an isolated anaemia remains unclear, it is worth classifying on the basis of the mean cell volume (MCV) as this helps to direct further investigation (Figure 1.1). Figure 1.1 Investigation of anaemia in critical care. MCV, mean cell volume; DIC, disseminated intravascular coagulation; TMAs, thrombotic microangiopathies; HLH, haemophagocytic lymphohistiocytosis; BM, bone marrow; AoCD, anaemia of chronic disease; PNH, paroxysmal nocturnal haemoglobinuria. Anaemia is most commonly normocytic in critically ill patients and usually multifactorial [4]. Iatrogenic reasons include frequent blood sampling and haemodilution (intravenous fluid administration). Occult blood loss can occur as a result of gastrointestinal mucosal inflammation and contributed to by coagulation defects, thrombocytopenia and uraemia. Another important reason is secondary anaemia, also termed anaemia of chronic disease (AoCD) or anaemia of inflammation. This is the most common cause of anaemia in hospitalized patients and can be caused by infection, cancer, autoimmune disease or chronic kidney disease [5]. This results in a functional iron deficiency, whereby adequate iron stores are present but the availability of iron for erythropoiesis is reduced. Hepcidin, a peptide produced by the liver in response to inflammatory cytokines, may be at least in part responsible for this phenomenon. Typically, ferritin is elevated and serum iron and transferrin saturation are low. Iron supplementation usually produces no improvement (in contrast to true iron deficiency). In addition to functional iron deficiency, inflammation results in reduced EPO production, reduced marrow sensitivity to EPO and shortened red cell survival. Check haematinics as combined deficiencies can result in a normal MCV. Additionally, due to the acute phase response, a normal ferritin does not exclude iron deficiency. Consideration should be given to the possibility of occult or recent bleeding. The anaemia is likely to resolve with improvement of the underlying conditions. Meanwhile, supportive management (including transfusion when required; see Chapter 17) is the mainstay of therapy, and blood sampling should be kept to a minimum. This is often a result of iron deficiency (blood loss, malabsorption or dietary deficiency). It can also occur during prolonged and severe illness as an extreme form of AoCD. Less commonly, microcytosis can be due to a haemoglobinopathy (e.g. thalassaemia trait), acaeruloplasminaemia, myelodysplasia, hyperthyroidism, lead poisoning and some rare congenital conditions (e.g. congenital sideroblastic anaemia).
Diagnostic Approach to Anaemia in Critical Care
Diagnostic approach to anaemia in critical care
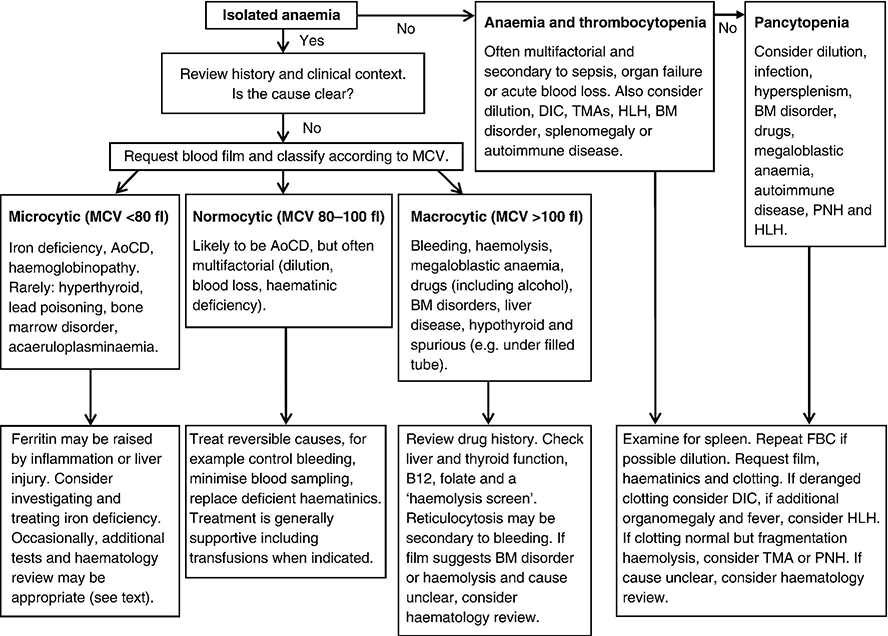
Normocytic anaemia (MCV within the normal range)
Microcytic anaemia (MCV below the normal range)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree