RATIONALE FOR DIAGNOSING MATERNAL HYPERGLYCEMIA
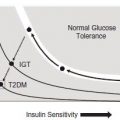
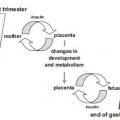
Gestational hyperglycemia as defined by criteria for “impaired glucose tolerance” accepted by the WHO2 o r as “gestational diabetes” by the ADA3 has been associated with stillbirth, fetal overgrowth, and injury at birth. The ADA defines gestational diabetes as “any degree of glucose intolerance with onset or first recognition during pregnancy”, but provides diagnostic thresholds for fasting and post-glucose loading values3 (Table 6.1). Gr avidas w ith even mild hyperglycemia are at increased risk of offspring with respiratory insufficiency, hypoglycemia polycythemia, and hyperbilirubinemia. The association of measures of maternal hyperglycemia with adverse perinatal outcomes, especially stillbirth, have been recognized for decades and continue to be identified in recent reports. In 1972, Karlsson and Kjellmer7 reported a four-fold increase in stillbirths in mothers with mean glucose values of 5.6 – 8.3 mmol/L (100 – 150 mg/dL) compared to those with lower mean values. O’ Sullivan et al4 chose statistical diagnostic criteria of maternal hyperglycemia (two or more of four values at ≥ 2 SD above the mean) from a 100- g 3-hour oral GTT. They were able to identify a subgroup of gravidas, older than 24 years of age, with a four-fold risk for stillbirth.8 Though O’Sullivan et al’s study sought diagnostic criteria predictive of later maternal Type 2 diabetes, their criteria, modified as to present assay techniques, have been adopted in the US to define gestational diabetes mellitus (GDM).3
More recent studies suggest that, despite improvements in obstetric care, gestational diabetes is still associated with increased stillbirth risk. Aberg et al studied the rate of stillbirth in prior pregnancies among Swedish women newly diagnosed with gestational diabetes in their subsequent pregnancy. They found a stillbirth rate of 14.9 per 1000 in the prior pregnancies (births from 1987 to 1992), a significantly increased risk (RR 1.6, 95% CI 1.1 – 2.2) compared to population rates.9 Obesity is well recognized to predispose to glucose intolerance. Examination of the association of maternal obesity with stillbirth has demonstrated an increased risk of stillbirth from an RR of 1.2 (95% CI 0.6 – 2.2) to 3.1 (95% CI 1.6 – 5.9) among gravidas with a body mass index (BMI) of 25 – 29 and 30 or greater compared to those with BMIs of 18.5–24.9.10 Cohorts from as late as the mid-1990s demonstrate continued increased risk of stillbirth, even among those diagnosed with GDM. Conde-Agudelo et al documented a 1.9-fold (95% CI 1.5 – 2.1) risk of stillbirth among gravidas with gestational diabetes compared to non-diabetic controls.11 Consequently, it may reasonably be inferred that subclinical maternal hyperglycemia during pregnancy predisposes to late fetal death, thereby emphasizing the importance of close glycemic monitoring in diabetic pregnancy.
Glycemic monitoring and liberal use of insulin to achieve maternal euglycemia has been associated with a fall in perinatal mortality rate. Beischer et al12 retrospectively examined a group of gravidas receiving GDM treatment (1981–1995) whose 75-g, 1-hour OGGT glucose values lay between 9 and 10 mmol/L (163 – 180 mg/dL) and 2-hour values between 7 and 7.8 mmol/L (127 – 140 mg/dL), and historical controls (1971 – 1980) within the same OGTT stratum who were not identified as having gestational diabetes and received only routine prenatal care. Those identified as having GDM had a perinatal mortality rate of less than one-third of the earlier control cohort (7 of 1000 vs 26 of 1000).
Observational studies have demonstrated an association between GDM and macrosomia at birth13,14 and randomized trials have demonstrated that treatment of even modest maternal hyperglycemia reduces fetal macrosomia.15,16 These data suggested that identification and treatment of women with mild degrees of glucose intolerance may reduce perinatal mortality and improve rates of perinatal morbidity.
Fetal overgrowth in the context of maternal gestational diabetes (ADA criteria) also appears to be a marker of later evidence of fetal imprinting that leads to later metabolic disorders. Offspring of mothers with GDM who demonstrate fetal overgrowth have been found to be at increased risk of obesity at 1 year of age.17 Further, offspring of glucose intolerant mothers (ADA criteria) with a birthweight above the 90th centile demonstrate a 3.6-fold risk of developing the metabolic syndrome (two or more of the following: obesity, hypertension [systolic or diastolic], glucose intolerance, and dyslipidemia) as early as 11 years of age compared to those with a normal birthweight.18
Because of the attribution of fetal risk to modest maternal hyperglycemia, oral glucose testing of gravidas for glucose intolerance became widespread. In the US, such screening has taken the form of a modification of the O’ Sullivan protocol, in which all patients were tested in the afternoon following a 50-g oral glucose load.4 Current US practice allows for testing at 24 – 28 weeks of gestation. The glucose load may be administered at any time of day, without regard to the time elapsed since the last meal. Plasma glucose is measured 1 hour after ingestion. A 100-g, 3-hour OGTT is recommended for those with a screening test value over 7.8 mmol/L (140 mg/dL). The original O’ Sullivan thresholds have been modified, based on present day use of glucose oxidase methods in venous plasma (Table 6.1).
The practice of screening for subclinical glucose intolerance has been controversial. Sermer et al19 blinded patients and caregivers to glucose testing results of gravidas meeting the later, lower-threshold criteria for gestational diabetes,20 but not the earlier, higher-threshold criteria.1 These subjects received usual prenatal care but were not identified as having gestational diabetes. Those subjects meeting the higher-threshold criteria underwent glucose surveillance and treatment. Compared with euglycemic controls, those (untreated) subjects meeting only the lower-threshold criteria had elevated rates of fetal macrosomia (28.7% vs 13.7%) and cesarean delivery (29.6% vs 20.2%). By contrast, gravidas meeting the higher (National Diabetes Data Group [NDDG]) threshold glucose criteria and who had been treated to achieve more normal glucose values, had a reduced rate of fetal macrosomia, comparable with euglycemic controls. However, despite normal fetal weight, those labeled with gestational diabetes had a cesarean rate of 33%, which was higher than that of untreated glucose-intolerant gravidas. These findings supported earlier recommendations of the US Preventive Services Task Force21 that screening for glucose intolerance in pregnancy should be abandoned because of a lack of demonstrated maternal or fetal benefit. The report suggested that a scientifically rigorous randomized clinical trial demonstrating perinatal benefit was required to justify a screening program.
In 2005, Crowther et al22 conducted a double blind, randomized trial of 1000 gravidas who were first identified by diabetes risk factors or by a 50-g, 1-hour glucose challenge (≥ 7.8 mmol/L [140 mg/dL]), and then diagnosed as GDM between 24 and 34 weeks of gestation by a 75-g, 2-hour GTT (WHO criteria: fasting plasma glucose value of < 7.8mmol/L [<140mg/dL]) and 2-hour post-load value of 7.8–11mmol/L (140–198mg/dL). Thus, the trial effectively excluded patients with significant degrees of glucose intolerance. Gravidas were randomized to intervention (diet counseling, glucose monitoring instruction, four-times daily glucose testing, and insulin treatment for repetitive values of over 5.5mmol/L [99mg/dL] fasting and 7.0mmol/L [126mg/ dL] 2 hours after meals) or to routine prenatal care. Prenatal care in the intervention group was that usually provided to women with GDM at each care site. Both the patients in the non-intervention group and their caregivers were blinded as to their having gestational diabetes. Despite the enrollment of only patients with mild glucose intolerance, 1% of offspring in the intervention group versus 4% of controls sustained serious perinatal outcomes (death, shoulder dystocia, fracture, or nerve palsy), a relative risk, adjusted for maternal age, race or ethnic group, and parity, of 0.33, but without a statistically significant difference in cesarean delivery rates (31% and 32%, respectively). There were fiveperinatal deaths among the controls, but none in those given diabetes care. This finding of efficacious pregnancy intervention among those with mild glucose intolerance changed the debate from that of whether screening for maternal glucose intolerance is justified to one addressing the best diagnostic threshold to be employed.
DIAGNOSTIC CRITERIA FOR MATERNAL HYPERGLYCEMIA
As noted above, O’ Sullivan et al4 employed the diabetogenic effect of pregnancy as a condition to predict later diabetes in women. They chose statistical diagnostic limits of greater than or equal to 2 SD above mean glucose values from a 100-g, 3-hour OGTT. O’Sullivan’s studies employed the Somogyi – Nelson method that identified other reducing substances in addition to glucose. As a result, glucose concentrations were elevated by approximately 0.28 mmol/L (5 mg/dL) over that determined by the more specific enzyme methods, now employed universally. Further, O’ Sullivan measured glucose in venous whole blood; measurements in plasma being approximately 14% higher. Subsequent studies have confirmed that more recent translation of O’Sullivan’s data accurately reflect his measures using glucose oxidase methods in venous plasma.20,23
The ADA 100-g, 3-hour OGTT, a transliteration of O’ Sullivan’s original test to modern laboratory values, is most commonly employed in the US. In 2003 – 4, it identified GDM among 4.2% of gravidas.24 Perinatal mortality risk among pregnancies with GDM (ADA criteria) remains at roughly twice that of pregnancies without diabetes. Recent reports of the prevalence of pre-eclampsia or pregnancy-associated hypertension among gravidas with GDM using ADA criteria have ranged widely. However, a summary of 10 reports of risk of these disorders in pregnancy with versus without GDM observed an overall increased risk of hypertensive disorders of only 8% among the total of over 4000 pregnancies with GDM.25
In 1991, Lind et al reported GTTs in 1009 unselected gravidas at more than 16 weeks of gestation.26 They pro-posed that values of 2 SD above the mean at fasting, 1 and 2 hours after a 75-g oral load (respectively 7, 11, and 9 mmol/L [126, 198, and 162 mg/dL]) be used for diagnostic thresholds; and that an elevated 2-hour and either an elevated fasting or 1-hour value be required to diagnose GDM. The use of these thresholds resulted in an incidence estimate of 1.2%, approximately one-third of that for the modified O’ Sullivan criteria. Correlation with perinatal morbidity was not provided.
Outside the US, a 75-g, 2-hour OGTT adopted by the WHO2 has been most commonly used to diagnose gestational diabetes. The test uses the same criteria to define gestational diabetes as are used to define impaired glucose tolerance in non-pregnant women, based on their association with diabetes-related morbidity. An abnormal test result requires only one abnormal value (Table 6.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree