Classification
Stage I
Involvement of a single lymph node region or lymphoid structure (e.g., spleen, thymus, Waldeyer’s ring) or involvement of a single extralymphatic site (IE)
Stage II
Involvement of two or more lymph node regions on the same side of the diaphragm (hilar nodes, when involved on both sides, constitute stage II disease): localized contiguous involvement of only one extranodal organ or site and lymph node region(s) on the same side of the diaphragm (IIE): the number of anatomic regions involved should be indicated by a subscript (e.g., II3)
Stage III
Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by involvement of the spleen (IIIs) or by localized contiguous involvement of only one extranodal organ site (IIIE) or both (IIIs E) III1: with or without involvement of splenic, hilar, celiac, or portal nodes
III2: with involvement of para-aortic, iliac, or mesenteric nodes
Stage IV
Diffuse or disseminated involvement of one or more extranodal organs or tissues, with or without associated lymph node involvement
Designations applicable to any disease stage:
A: No symptoms
B: Fever (temperature above 38 °C), drenching night sweats, unexplained loss of more than 10 % of body weight within the preceding 6 months
X: Bulky disease (a widening of the mediastinum by more than one-third or the presence of a nodal mass with a maximal dimension greater than 10 cm)
E: Involvement of a single extranodal site that is contiguous or proximal to the known nodal site
CS: Clinical stage
PS: Pathological stage (as determined by laparotomy)
In 1998 an International Prognostic Factor Project on advanced Hodgkin’s disease was developed based on data from 1618 patients treated at 25 centers [46]. The prognostic score was defined as the number of adverse prognostic factors at diagnosis (Table 45.2). Whether or not this prognostic score will be routinely used in making therapeutic decisions, as is the case for the International Prognostic Index in non-Hodgkin’s lymphoma [47], is still uncertain [48].
Table 45.2
The Hasenclever International prognostic score for advanced stages
Independent variable with negative prognostic significance | |
|---|---|
Variable | Value |
Albumin | <4 g/dl |
Hemoglobin | <10.5 g/dl |
Sex | Male |
Age | ≥45 years |
Stage | IV |
Leukocytosis | White blood cells >15,000/mm3 |
Lymphocytopenia | Absolute lymphocyte count <600/mm3 or <8 % total white blood count |
Rates of freedom from progression and overall survival at 5 years according to individual and grouped prognostic scores | |||
|---|---|---|---|
Prognostic score | N patients (%) | Rate of FFP (%) | Rate of OS (%) |
Single negative factors | |||
0 | 115 (7) | 84 ± 4 | 89 ± 2 |
1 | 360 (22) | 77 ± 3 | 90 ± 2 |
2 | 464 (29) | 67 ± 2 | 81 ± 2 |
3 | 378 (23) | 60 ± 3 | 78 ± 3 |
4 | 190 (12) | 51 ± 4 | 61 ± 4 |
5–7 | 111 (7) | 42 ± 5 | 56 ± 5 |
Grouped negative factors | |||
0 or 1 | 475 (29) | 79 ± 2 | 90 ± 2 |
2 or more | 1,143 (71) | 60 ± 2 | 74 ± 2 |
0 to 2 | 939 (58) | 74 ± 2 | 86 ± 2 |
3 or more | 679 (42) | 55 ± 2 | 70 ± 2 |
0 to 3 | 1,317 (81) | 70 ± 2 | 83 ± 1 |
4 or more | 301 (19) | 47 ± 2 | 59 ± 2 |
Recommended Pretreatment Evaluation
Table 45.3 outlines the procedures deemed necessary for correct staging of Hodgkin’s disease. An adequate surgical biopsy, possibly of more than one intact lymph node, should be undertaken for pathological examination. Inguinal nodes should not be biopsied if other equally suspicious peripheral nodes are present. When the diagnosis of Hodgkin’s disease is made from biopsy of an extranodal site, a concomitant nodal biopsy for confirmation of diagnosis is desirable.
Table 45.3
Recommended procedures for proper staging
Adequate surgical biopsy reviewed by an experienced hemopathologist: in primary extranodal lymphomas, biopsy should also include a lymph node when palpable |
Detailed history with special attention to the presence or absence of systemic symptoms |
Careful physical examination, emphasizing node chains, size of liver and spleen, Waldeyer’s ring inspection, and bony tenderness |
Routine laboratory tests: complete blood count, erythrocyte sedimentation rate, liver function tests, serum uric acid, serum lactate dehydrogenase |
Chest radiograph (posteroanterior and left lateral) with measurement of mediastinal mass/thorax ratio |
Bipedal lymphangiography in selected cases if adequately trained radiologists are available |
Chest and abdominal CT scan and abdominal ultrasound |
Radioisotopic evaluation with 67gallium especially if mediastinum is involved |
Unilateral bone marrow biopsy in stages III-IV and any stage with B symptoms |
Cytologic examination of any effusion |
18Fluorodeoxyglucose positron emission tomography in suspicious cases |
Any further study when suggested by clinical symptoms or sign (e.g., bone scan) |
A detailed history must be obtained with information about the presence or absence of unexplained fever and its duration, unexplained sweating (especially at night) and its severity, unexplained weight loss as a percentage of usual body weight and rapidity of loss, and pruritus with its extent and severity. The presence of alcohol-induced pain, a family history of Hodgkin’s disease or other hematologic malignancy, any history of immunosuppressive illness, such as infection with human immunodeficiency virus (HIV), or EBV infection, such as mononucleosis or a previous history of neoplasm together with any previous chemotherapy or radiation therapy should also be documented. A careful and complete physical examination must be performed with special attention to the number of enlarged lymph nodes and their dimensions, presence or absence of enlarged liver or spleen involvement, and Waldeyer’s ring involvement. A complete laboratory work-up is required.
A variety of imaging techniques are available to assist in the initial staging of patients with histologically documented, previously untreated Hodgkin’s lymphoma [49–56]. The usefulness of a specific imaging test can be defined in terms of sensitivity, specificity, and overall accuracy. The following guidelines are suggested:
1.
Thorax evaluation should include chest radiograph and thoracic CT scan to evaluate mediastinal adenopathy and any extension of such adenopathy into the surrounding viscera. In addition, it is important to assess the bulk of disease, which is of well-documented prognostic significance. Furthermore, PET scanning should be performed at diagnosis at least in all patients with mediastinal involvement in order to better evaluate the nature of residual abnormalities at the end of the treatment program [57–59].
2.
Abdominal involvement must be evaluated by CT scan, which is particularly helpful in evaluating upper abdominal nodal disease and can detect enlargement of celiac, portal, splenic hilar, and mesenteric nodes as well as retroperitoneal nodes. Bipedal lymphangiography is still recommended in centers with adequately trained radiologists for its unique capability to describe the lymph node architecture even in the absence of pathologic enlargement. However, despite the fact that lymphangiography remains the most accurate diagnostic tool for assessment of low para-aortic and paracaval nodal disease, its use has been largely abandoned because of the time and effort the procedure requires relative to CT and MRI scanning. Nevertheless, in adequately equipped centers, CT scan and lymphangiography should both be used in selected patients for their complementary information.
3.
Bone marrow should be studied by core needle biopsy from the posterior iliac crest in patients with CS III or IV. It is not necessary in early-stage patients without B symptoms.
4.
In all cases, suggestive symptoms and signs of involvement at any specific site should prompt the performance of specific diagnostic studies.
Radiolabeled fluorodeoxyglucose positron emission tomography (18FDG-PET) has emerged as a very useful tool in evaluating the presence of active neoplastic tissue in residual masses after therapy, especially in the infradiaphragmatic areas. By utilizing the 18FDG metabolism of neoplastic cells, 18FDG-PET scanning seems to be able to recognize small lesions (less than 1 cm in diameter), with a high sensitivity approaching 100 % despite a low specificity (slightly superior to 70 %). PET scanning may reveal involved areas undetectable with other imaging techniques [60]. It should be noted, however, false-positive PET scans are not uncommon [61].
The biopsy of specific sites to determine involvement is of advantage in some situations. A percutaneous needle biopsy of the liver is frequently negative even when disease was readily detectable at laparotomy in days gone by, due to the focal pattern with which Hodgkin’s lymphoma involves the liver. A percutaneous or laparoscopic liver biopsy can be helpful in patients with abnormal liver function tests or equivocal radiographic studies of the liver. A guided bone biopsy using CT or a percutaneous or open-lung biopsy may be indicated under similar circumstances when evidence of involvement is otherwise equivocal. In prescribing such invasive procedures, the physician should always consider if a positive result would modify the treatment approach.
The Purpose of Pretreatment Staging
The inclusion of chemotherapy in the treatment of virtually all cases of Hodgkin’s lymphoma has radically modified the pretreatment work-up. The abandonment of staging laparotomy is a direct consequence of this new role of chemotherapy. Staging laparotomy and splenectomy was aimed at identifying occult stage III patients unsuitable for radiotherapy alone programs, whereas negative cases were considered candidates for nodal irradiation alone [62, 63]. At present only stage IA patients with no adverse factors are properly treated with irradiation alone, and even in this case many centers prefer to prescribe brief chemotherapy and involved-field irradiation or even chemotherapy alone. Furthermore, the diagnostic capability of modern imaging techniques has been progressively refined, which reduces the need for a surgical diagnostic approach.
In planning the diagnostic work-up for a patient with Hodgkin’s lymphoma not involved in a clinical trial, one should consider how the results of each examination would change the therapeutic decision. For example, if the abdominal CT scan shows infradiaphragmatic involvement (at least stage III disease), the treatment program does not generally differ on the basis of other possible sites involved. Thus, the need for other invasive and/or expensive procedures, such as gallium scanning, lymphangiography, and PET scanning can probably be avoided. Otherwise, in the presence of a negative abdominal CT scan, other tests, such as lymphangiography, and/or PET scanning, can help in identifying occult involvement, which will change the treatment decision, primarily with respect to the duration or intensity of chemotherapy. In the same case, a PET scan may be useful in evaluating therapeutic response. Some investigators argue that PET scans have limited clinical impact in patients with Hodgkin’s lymphoma [64] and false-positive scans after treatment are not uncommon [65]. Others find them very useful in assessing response to therapy [66]. Figure 45.1 shows the minimal work-up required to adequately stage a patient with a new diagnosis of Hodgkin’s disease.
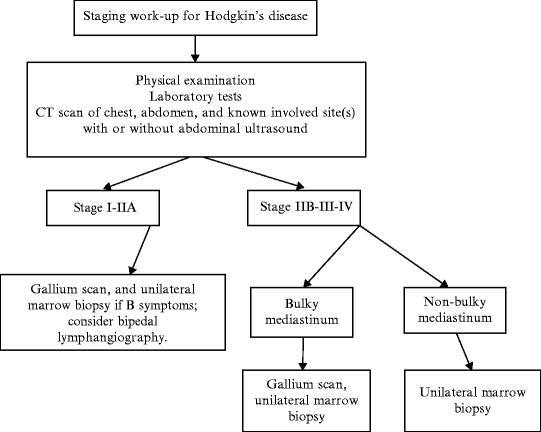

Fig. 45.1
Minimal staging for patients outside of a clinical trial
Evaluation of Response and Follow-Up Studies
After completion of planned therapy, response should be documented on the basis of clinical findings, and the results of repeat imaging studies that were abnormal at presentation, supplemented by tissue biopsies if necessary. A number of patients have residual abnormalities after therapy, typically with mediastinal widening. It is well known that residual shadows on imaging, especially in the mediastinum do not necessarily represent residual disease but rather may indicate necrosis or fibrosis, and abnormal radiographic findings may persist for months to several years in such patients who are otherwise without evidence of disease [57, 58]. This situation, defined as CRu, denotes patients in whom response status is unclear. The patient with CRu is in normal health with no clinical evidence of Hodgkin’s disease but with some radiologic abnormality, consistent with the effect of therapy, persisting at a site of previous disease. Implicit in this designation is a considerable uncertainty about the significance of such abnormalities. Attempts to resolve the dilemma of persistent disease versus residual anatomic distortion not indicative of Hodgkin’s lymphoma should include repeated investigations, such as radiologic imaging in a few months, or biopsy. Within the bounds of acceptable morbidity, pathologic examination such as mediastinoscopy may be appropriate, although the difficulties of sampling artifacts should be kept in mind. It is highly recommended that a second biopsy of liver, bone marrow or both be performed when these sites were initially involved.
After completion of therapy, it is recommended that patients be seen at 2 month intervals during the first and second post-treatment years, at 4–6 month intervals from the third to fifth year, and annually thereafter. The frequency and type of radiologic follow-up studies depend and should focus on the initial sites of disease, as well as the detection of medium and long-term sequelae of treatment. Appropriate workup should be initiated when symptoms or signs of possible recurrent disease are noted. Because of the relatively high incidence of second neoplasms, investigations useful for the early detection of new malignancies, particularly breast and lung cancer should be part of the follow-up studies for patients in remission [67, 68]. Screening mammography or breast ultrasound, even in young women treated by mantle-field irradiation, is an example of this policy [68].
Treatment
Evolution of the Treatment Strategy
Treatment of Hodgkin’s lymphoma has continuously improved since the 1960s and 1970s, and today about 85 % of all patients can be cured by first- or second-line therapeutic strategies [69–71]. Although its cell of origin, etiology, and pathogenesis remain to be further elucidated, Hodgkin’s disease represents a remarkable example of how progress in clinical research can outpace progress in basic research. The continuous improvement in prognosis has been the result of meticulous clinicopathologic observations and sound prospective trials (Table 45.4).
Table 45.4
Chronologic flow of major concepts and events influencing treatment evolution of Hodgkin’s disease
Investigator(s) (years) | Major concepts and events |
|---|---|
Gilbert (1925–1939) | Concept of destruction of all lesions with the first course of radiation therapy: segmental irradiation to encompass suspected microscopic disease |
Peters (1950–1958) | Improved 5- and 10-year survival by prophylactic irradiation of adjacent lymphoid areas: first three-stage clinical classification |
Kinmoth (1952) | Lower extremity lymphangiography |
Easson and Russel (1963) | Concept of cure by radiation therapy |
Lukes (1963–1964) | Relationship of histologic features to clinical stages and prognosis |
Kaplan (1962–1965) | Development of wide-field technique with radiation in continuity with multiple-node chains (mantle, inverted Y, and total lymphoid radiation therapy): identification of tumoricidal dose levels |
Rosenberg and Kaplan (1965) | Evidence for an orderly progression in the spread of Hodgkin’s disease |
Lacher and Durant (1965) | Increased complete remission rate by vinblastine plus chlorambucil compared with single agents |
Frei (1966) | Efficacy of a cyclic four-drug combination (MOPP) |
Kaplan and Glatstein (1969) | Staging laparotomy and further studies on the pattern of anatomic distribution |
DeVita (1970–1980) | Concept of high cure rate in advanced disease with MOPP |
DeVita, Young (1971–1973) | Staging laparoscopy—no advantage of maintenance chemotherapy in complete responders |
Rosenberg (1968–1981) | Trials with combined radiation therapy and chemotherapy, especially MOPP |
Wiernik (1968–1985) | Trials of combined-modality therapy with MOPP and radiation therapy |
Bonadonna and Santoro (1973–1987) | Development of non-cross-resistant chemotherapy (ABVD) and of alternating regimens (MOPP/ABVD) |
Wiernik (1976) | Randomized trial of MOPP versus combined-modality therapy for early-stage disease |
Longo et al., Cimino et al. (1982–1990) | Randomized trials of MOPP versus radiation therapy alone for early-stage Hodgkin’s disease |
Canellos, Connors, Viviani et al. (1980–1998) | Randomized trials comparing MOPP, ABVD, and hybrid or alternating MOPP/ABVD in advanced stage |
Santoro et al., Horning et al. (1990–1997) | Low-toxic brief chemotherapy (ABVD, VBM) and involved-field radiotherapy for early-stage Hodgkin’s disease |
Horning, Chopra, Sweetenham Josting et al. (1990–2000) | Studies with high-dose chemotherapy and bone marrow transplantation for salvage therapy |
Diehl, Horning, Gobbi, Wiernik | New-generation intensive regimens (BEACOPP, Stanford V, MOPPEBVCAD, MVC) for advanced stages and salvage therapy |
Radiation therapy progressively developed following the work of Gilbert (1939) and Peters (1950) and became a major treatment for lymphoma with the beginning of the megavoltage era. Studies by Kaplan and the Stanford group [23] led to the development and refinement of many concepts and techniques that constitute modern radiation therapy for lymphomas and particularly for Hodgkin’s lymphoma [72–75]. Until the end of the 1960s, radiation therapy was the only successful therapeutic tool that, under appropriate circumstances, was able to achieve cure in a sizeable fraction of patients with Hodgkin’s lymphoma. Chemotherapy was developed after the end of World War II, and lymphomas and leukemias were among the first neoplasms shown to respond to drug treatment. Although the great majority of available cytotoxic agents can produce objective responses in Hodgkin’s lymphoma, it was only in 1970 with the four-drug regimen known as MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) that DeVita and Carbone documented that chemotherapy could induce durable complete remissions and cure with high frequency in advanced Hodgkin’s lymphoma [76].
In the late 1970s and 1980s, radiation therapy and chemotherapy were sequentially combined for the treatment of early as well as advanced stages to maximize relapse-free survival (RFS), and to improve the cure rate. In an attempt to eradicate both MOPP-sensitive and MOPP-resistant neoplastic cells, Bonadonna et al. designed the ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine) regimen and mounted a number of prospective trials that proved its effectiveness as a non-cross-resistant regimen to MOPP [77–82]. Further attempts to improve the cure rate took into consideration the Goldie–Coldmann hypothesis [83] by testing the effect of early introduction of as many effective drugs as possible in treatment strategies. Trials from the Milan, Vancouver, and other groups compared alternating MOPP/ABVD with the so-called hybrid regimen which alternates half MOPP and half ABVD [84–86]. Actually, the hybrid regimen or MOPP alternating with ABVD has not demonstrated any real advantage over ABVD alone [87].
In 1992 Canellos and colleagues from the Cancer and Leukemia Group B (CALGB) confirmed in a large randomized study that ABVD alone was superior to MOPP. Since then, ABVD chemotherapy has become the gold standard against which all newer regimens should be tested [88].
In the 1990s, further efforts to improve the outcome of patients with Hodgkin’s disease have consisted of the intensification of primary chemotherapy with hematopoietic colony-stimulating factor (CSF) support, with the aim both to increase complete remission rate and to minimize late toxic effects. A major example is the BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) [88, 89], designed by Diehl and coworkers from the German Hodgkin’s Study Group, as well as of the Stanford V regimen of Horning et al. (weekly alternation of doxorubicin, vinblastine, mecloretamine, vincristine, bleomycin, etoposide, plus prednisone) [90, 91], and the Italian MOPPEBVCAD (mechloretamine, CCNU, vindesine, melphalan, prednisone, epiadriamycin, vincristine, procarbazine, vinblastine, bleomycin) [92]. Recently, it was demonstrated that the Stanford V regimen is not superior to ABVD in any outcome measure [93].
The fundamental question of whether a clinical dose–response effect can be exploited with the use of high-dose chemotherapy with autologous [93–98] and allogeneic [99–101] stem cell rescue has been tested worldwide as salvage therapy for relapsed or refractory patients. Since initial attempts in the early 1980s, numerous investigators used high-dose chemotherapy with autologous bone marrow transplantation (ABMT) or peripheral blood stem cell transplantation (PBSCT) for salvage treatment of patients with relapsed Hodgkin’s disease. Despite the publication of only one randomized comparison between high-dose and standard-dose chemotherapy [98], a consistent proportion of patients has been cured at relapse with this high-dose strategy. Thus, the trend toward a better outcome for patients treated by high-dose chemotherapy for relapse, along with the low morbidity rate, now reported as <1–4 % for autologous and 10–20 % for allogeneic transplants confirms the validity of these approaches.
Improvement in treatment results has been achieved in all stages of the disease. Today, no stage is beyond cure when treated appropriately at the time of diagnosis or when treated for recurrence. This represents a dramatic improvement in the overall prognosis of a disease that 40 years ago was considered to be almost universally fatal.
General Treatment Strategy
Multiple trials have identified a variety of treatment options, most of which appear to be relatively beneficial. In other words, in many disease stages a choice of treatment exists rather than a treatment of choice. The cost/benefit ratio is an important component in the selection of a specific therapy. Regardless of treatment options, several factors need to be remembered (1) total tumor burden and optimal dose delivery remain the most important treatment variables; (2) primary tumor cell resistance represents the major stumbling block for all chemotherapy regimens; and (3) not only are prospective randomized trials (often repetitive) difficult and lengthy undertakings, but their influence on current clinical practice may actually be limited when patient accrual continues indefinitely. In fact, new concepts in staging, treatment, or both may develop outside the context of such a trial and may affect its relevance [71].
As already mentioned, staging laparotomy has been abandoned and lymphangiography has been progressively less used as consequences of the advent of sophisticated imaging techniques, such as CT and PET scanning, and of the widespread use of chemotherapy in virtually all lymphoma stages. However, careful patient selection remains important to identify those who will benefit from each treatment strategy. Some major considerations should be taken into account when decision making is needed for an individual patient, especially concerning the multimodality approach, such as the following:
1.
Despite a multitude of publications concerning further prognostic classification on the basis of clinical and biological features [62], the Rye–Ann Arbor–Cotswolds staging system currently provides the best methodology for discriminating between early and advanced stages.
2.
In early stages (I, IIA), the multidisciplinary approach is now considered standard, but is seriously challenged by data on chemotherapy alone for early patients [102–110]. As the vast majority of these patients (approximately 90 %) can be cured, the choice of treatment is presently aimed at reducing both acute and late toxicities by introducing less toxic drugs and reducing the dose and extension of radiation fields, or eliminating radiation therapy altogether.
3.
In patients presenting with massive mediastinal involvement, the role of combined-modality therapy is well established in all stages of disease [108].
The usefulness of consolidation radiotherapy to all sites of disease has long been a matter of debate. Some groups prefer to irradiate all nodal sites of disease at diagnosis even if the advantage of this approach is not fully demonstrated [110]. It is likely that irradiating all nodal areas in a patient with extensive stage IV disease will not enhance the probability of cure, whereas radiotherapy may be advantageous in those patients with advanced, exclusively nodal disease, but not more effective than continuing chemotherapy.
Although improved in the last 25 years, salvage treatment is curative only in about one-half of relapsed patients, except under the most favorable circumstances, such as limited-stage relapse beyond the 12th month of initial complete remission [113, 114]. As pointed out by Rosenberg [115] new salvage therapies should not be evaluated hastily since curability and comparisons of toxicities require 5–10 years of careful evaluation.
Nearly one-third of patients with Hodgkin’s lymphoma die without evidence of lymphoma at autopsy, and in a significant number of patients, death is attributable to complications of therapy [115].
Therefore, treatment programs should be designed not only to improve therapeutic results but prevent or reduce iatrogenic complications such as cardiac and neoplastic as well. Long-term complications of treatment are discussed next.
Treatment of Early Stages (IA–B, IIA)
The vast majority of patients with early-stage Hodgkin’s disease (IA–IIA) can be cured by currently available therapeutic options. Thus, a major aim of treatment is to limit medium- and long-term toxic effects rather than to further increase the cure rate. However, it should be noted that there is no complete agreement on the definition of early stages, as some authors include cases with bulky disease or B symptoms, whereas others do not (Table 45.5). As a consequence, it is somewhat difficult to draw precise conclusions from published series concerning the same treatment modalities but with different patient selection criteria.
Table 45.5
Prognostic subgroups in stage I–II
EORTC | |
|---|---|
Very favorable | CS IA, female sex, age <40, and LP histology, or NS histology and nonbulky mediastinum |
Favorable | Cases classifiable neither as very favorable nor as unfavorable |
Unfavorable | One of the following factors: bulky mediastinum, stage A with ESR >50, B with ESR >30, age >50, more than four sites involved |
GHSG | |
Unfavorable | Bulky mediastinum or elevated ESR, or |
•Three involved sites, or | |
•Disseminated extranodal or splenic involvement | |
Favorable | All others |
Stanford | |
Very favorablea | CS I and female sex, or |
CS I mediastinum, or | |
CS I and male sex with LP histology, or | |
CS II, female sex, age <27, and <three involved sites | |
Harvard | |
Very favorable | CS IA and female sex, or |
CS IA and male sex with LP histology, or | |
CS IA and male sex with peripheral cervical site | |
However, it seems clear that radiation therapy alone or chemotherapy alone can cure over 90 % of patients with Stage IA or IIA without bulky mediastinal disease, and that patients with bulky mediastinal disease require combined-modality therapy for optimal results.
Radiotherapy
Up to the 1980s, the standard approach to stage IA–IIA Hodgkin’s disease included staging laparotomy and splenectomy and, if these procedures are negative, mantle-field irradiation at therapeutic doses (36–44 Gy), followed by irradiation of para-aortic nodes and the splenic pedicle (subtotal nodal irradiation, STNI) at lower doses (30–36 Gy). With this approach, up to 90 % of patients were alive at 10 years with a freedom from progression of 70–80 % in one study [116]. Relapsing patients could, in fact, obtain second durable remissions by salvage chemotherapy. This approach has been reconsidered by a number of research groups. First, many radiation therapists documented a relapse rate in stage IIA that was at least double that initially reported, and in stage IIB patients a failure rate of about 50 % was observed with radiation alone [102, 117, 118]. However, it should be pointed out that selection criteria often included the so-called unfavorable early stages as outlined by the European Organization for Research and Treatment of Cancer (EORTC): MC and LD histologies, male sex, age older than 40 years, erythrocyte sedimentation rate more than 70, and mediastinal mass. In the presence of one or more unfavorable features, patients treated with extensive radiation therapy alone had a 6-year RFS of 53 % versus nearly 100 % when treated with combined-modality therapy [118]. Furthermore, the Stanford group tested subtotal nodal irradiation versus involved-field irradiation plus six cycles of MOP(P) in pathological stages IA–IIA, and concluded that adjuvant chemotherapy could replace radiation of occult disease [118]. The results of a number of major trials comparing radiotherapy, chemotherapy, and combined-modality therapy are shown in Table 45.6.
Table 45.6
Randomized trials comparing combined modality versus radiotherapy or chemotherapy alone in early stage Hodgkin’s disease
Author (ref) | Regimen | RT | Stages | FFP (%) | OS (%) | Median FU (years) |
|---|---|---|---|---|---|---|
Pavlovsky [119] | CVPP + RT vs. CVPP | IF | Unfavorable I–II | 75 | 84 | 7 |
34 | 66 | |||||
Horning [120] | VBM + RT vs. RT | IF | I–IIA, IIB, IIA favorable | 95 | 95 | 3 |
STNI/TNI | 70 | 67 | ||||
Radford [121] | VAPEC-B + RT vs. RT | IF | 91 | NR | 3.3 | |
I–IIA | ||||||
STNI | 73 | NR | ||||
Santoro [122] | ABVD/IF vs. ABVD/STNI | IF | 95 | 100 | 3 | |
I-IIA | ||||||
STNI | 94 | 100 | ||||
Wiernik [102] | MOPP + RT vs. RT | EF | IA, IIA, IIB, IIIA | 94 | 91 | 5.8 |
66 | 76 |
Over the last decade, indications for radiotherapy in early-stage Hodgkin’s disease have been progressively revised. At present, only a limited-stage IA presentation with no adverse factors is considered to be appropriately treated with mantle-field or subtotal nodal irradiation alone. However, as noted previously, the trend is toward chemotherapy alone even for these patients. Radiation therapy for Hodgkin’s lymphoma is discussed extensively in Chap. 46.
Chemotherapy
In the second half of the 1980s chemotherapy was introduced in the management of early-stage Hodgkin’s lymphoma. Some groups have tested the efficacy of chemotherapy alone in this setting in order to eliminate late effects related to combined-modality therapy. Two groups published randomized comparisons between MOPP chemotherapy and radiotherapy, reaching opposite conclusions. The Italian group [123] found that radiation therapy was ineffective as salvage treatment after MOPP failure; thus in their opinion, radiation therapy remains the treatment of choice. The National Cancer Institute (NCI) group observed a superiority of MOPP chemotherapy over radiation therapy and concluded that MOPP was at least as effective as radiation therapy [103].
The National Cancer Institute of Canada and ECOG compared two courses of ABVD followed by radiotherapy with four to six courses of ABVD alone. After a median of 4.2 years PFS was better for patients who received radiation therapy. Progression after ABVD alone was more frequent in sites that would have been irradiated in the other arm of the study, but there was no difference between the arms with respect to freedom from second progression or death rate [124]. Therefore, patients who progressed after initial treatment with ABVD alone could be successfully treated with salvage radiation therapy if required.
Combined Modality
Most trials of the last 20 years have focused on the role of a combined chemotherapy–radiotherapy strategy in early-stage Hodgkin’s disease. The debate arises from the evidence that salvage chemotherapy in patients relapsing after radiotherapy alone offers cure rates as high as initial combined-modality therapy. On the other hand, delivering chemotherapy after extensive radiotherapy means a higher risk of second malignancies. At the Milan Cancer Institute, 116 patients with pathologically staged favorable presentation who were treated with extended-field radiotherapy were non-randomly compared with 85 patients with unfavorable presentations who were treated with MOPP and radiotherapy. At 10 years, the freedom from progression was significantly different between stages I and II in the favorable group (85 versus 59 %) respectively, whereas stage lost its prognostic significance in the unfavorable group treated with combined-modality therapy [125]. Furthermore, in the favorable group a higher incidence of acute leukemia as a consequence of salvage chemotherapy was observed.
At Stanford, 67 patients with favorable pathological stages I and II (A and B), or IIIA were randomized between subtotal or total nodal irradiation alone or involved-field irradiation plus six cycles of the VBM regimen (vinblastine, bleomycin, methotrexate). At 5 years, freedom from progression was 70 % after radiation alone and 95 % after combined-modality treatment (p < 0.0001) [120]. In a subsequent trial (Stanford Permanente), the authors focused on favorable clinical stages I and II: 78 patients were randomized to receive either extended-field irradiation (subtotal nodal) or involved-field irradiation after VBM. With a median follow-up of 4 years, 92 % of the extended-field group and 87 % of the combined-modality group were free of progression. The VBM regimen is very well tolerated with little adverse effect on male or female fertility, and more than 90 % of the planned dose can usually be delivered. Despite the fact that comparable results between the two groups would support the use of combined-modality therapy in all patients, the authors did not recommend its routine use outside of a clinical trial [121].
The Milan group compared two different radiotherapy strategies following primary chemotherapy by randomizing 103 patients to receive STNI or involved-field radiotherapy after four courses of ABVD. In a preliminary report, with a median follow-up period of 38 months, the response rate was 100 %, freedom from progression was 95 %, and survival was 100 % without any difference between the two groups [122].
Thus, following complete remission with short-term chemotherapy, involved-field consolidation radiotherapy seems to offer good results with minimal toxicity.
In a meta-analysis, the role of adjuvant chemotherapy was assessed in 13 randomized trials involving 3,888 early-stage patients. The addition of chemotherapy to radiotherapy halved the 10-year risk of failure (16 versus 33 %), with a small statistically insignificant improvement in survival (79 versus 76 %). A reduction of Hodgkin’s disease deaths of borderline significance (12.3 % versus 15.4 %) was partly counterbalanced by an insignificant increase in deaths from other causes (12 versus 10 %) [126]. However, it could be argued that this meta-analysis refers to studies begun in the 1970s or early 1980s, when MOPP was the most frequently used combination. Thus, it is possible that less toxic regimens such as ABVD or VBM will reduce mortality from causes other than Hodgkin’s disease and establish the combined-modality advantage. In another meta-analysis, Loeffler et al. [127] concluded that combined-modality therapy for advanced-stage disease gave inferior survival outcome compared with chemotherapy alone. In summary, at present the optimal initial treatment for early-stage Hodgkin’s lymphoma is not known. Radiation alone, chemotherapy alone, or combined-modality therapy all have their advocates [128].
Nodular lymphocyte predominant Hodgkin’s lymphoma patients need to be considered differently with respect to treatment from classical Hodgkin’s lymphoma patients. Limited-field radiotherapy is considered standard for early stage disease (>90 % of patients), since it has been shown to be as effective in all outcome measures as combined-modality therapy and more effective than chemotherapy alone [129, 130]. However, recent studies have demonstrated efficacy for rituximab in this Hodgkin’s variant whose Reed–Sternberg cells are CD20+ [37]. New regimens that include rituximab are currently in clinical trial for this entity.
Treatment of Advanced Stages
Independent of the Hasenclever Prognostic Score, all stage IIB, III, and IV patients according to the Ann Arbor staging system are considered to be advanced. There is no consensus in the literature concerning stages IIAX and III1A, which are considered early stages by some investigators and advanced stages by others.
Conventional Approach
Primary chemotherapy is the standard approach for advanced stages. Up to the 1990s, the gold standard was the MOPP regimen [131], which induced complete remission in approximately 80 % of patients. Of those, approximately 30 % relapsed within 5–10 years.
It has become clear that MOPP alone is inferior to ABVD alone in the control of Hodgkin’s disease. The first trial demonstrating the superiority of an ABVD-based regimen over MOPP was conducted at the Milan Cancer Institute in patients with stage IV disease. The alternating MOPP–ABVD regimen demonstrated a 15–20 % advantage over MOPP [79]. Subsequently, the same group [81] demonstrated a significant advantage in terms of complete remission rate, freedom from progression, and RFS favoring ABVD (three courses followed by extended-field radiotherapy and three more courses) as compared with MOPP (same schedule) in 232 pathologically staged IIB and III patients. The comparable overall survival in the two groups was probably related to the fact that salvage ABVD was given to MOPP-resistant patients. The long-term results of this trial confirmed the efficacy of a combined-modality approach. As far as delayed toxicity is concerned, no signs of myocardial damage were seen in either arm. However, other studies with longer follow-up have reported a 10-year incidence of 5.5 % cardiac disease-related hospitalizations after ABVD alone and 9.2 % after ABVD plus mediastinal irradiation, compared with a 2.2 % rate expected in the general population [132]. The ABVD group showed more pulmonary radiation fibrosis than the MOPP group, in particular after the last three cycles of bleomycin-containing chemotherapy. Permanent testicular failure occurred only after MOPP. Although MOPP was definitively more leukemogenic than ABVD, the frequency of solid tumors was about the same with the two combinations [133]. Clearly, the incorporation of alkylating agents (mechlorethamine and procarbazine) in the MOPP regimen is responsible for the increased incidence of sterility and leukemogenesis observed with that treatment.
In the CALGB study comparing MOPP, ABVD, and MOPP alternating with ABVD in 362 patients with advanced-stage disease (IIIA2, IIIB, IV), 8-year freedom from progression was 37 % for MOPP, 52 % for ABVD, and 50 % for alternating MOPP and ABVD. A similar difference in overall survival did not emerge. A 3 % incidence of fatal febrile neutropenia in the MOPP group was counterbalanced by a similar incidence of fatal pulmonary toxicity with ABVD, and 1 % of each after MOPP–ABVD [134].
Some groups have tested the alternating MOPP/ABVD with the so-called hybrid MOPP/ABVD, which consists of alternating half courses of MOPP with half courses of ABVD, with the aim of more rapid delivery of all active drugs in concert with the Goldie–Coldman hypothesis. In the Vancouver trial, alternating MOPP and ABVD was compared with hybrid MOPP–ABV, with ABV given on day 8 of each 28-day course. Both regimens produced excellent results, but the hybrid regimen was associated with a higher incidence of life-threatening febrile neutropenia and stomatitis, leading to a lowering of the upper age limit from 65 to 55 years in mid-study [84]. In 415 patients treated at the Milan Cancer Institute, alternating and hybrid programs (half MOPP and half ABVD every 14 days of each 28-day cycle) produced comparable results [85] in terms of both survival and acute toxicity.
The North American Intergroup (CALGB, ECOG, SWOG) [86], compared sequential MOPP followed by ABVD with hybrid MOPP/ABV in 737 patients with advanced-stage (III2A, IIIB, IV) Hodgkin’s disease with the aim of testing the Goldie–Coldman hypothesis by introducing doxorubicin late (sequential) or early (hybrid) in the therapeutic program. Patients assigned to the sequential schedule received six MOPP courses followed, if complete response was attained, by three ABVD courses. Patients in the hybrid group received MOPP–ABV as designed by Connors et al. [84]. Complete remission was achieved in 83 % of cases on the hybrid and 75 % in the sequential group (p = 0.02). After a median follow-up period of 7.3 years, the 8-year FFP was 64 % for MOPP/ABV and 54 % for MOPP → ABVD (p = 0.01). The 8-year OS rate was significantly better for MOPP/ABV as compared with MOPP → ABVD (79 versus 71 %, p = 0.02). Acute life-threatening (grade 4) or fatal (grade 5) toxicity was more frequent in the hybrid regimen. However, seven cases of acute myeloid leukemia and two of myelodysplasia were diagnosed in patients receiving MOPP → ABVD as compared with one case of acute myeloid leukemia in the hybrid regimen (p = 0.01). Overall (Table 45.7), six to eight courses of ABVD alone has emerged as the gold standard in advanced-stage Hodgkin’s disease, with which new-generation regimens should now be compared.
Table 45.7
Therapeutic outcome at 5 or more years with MOPP, ABVD, or alternating/hybrid MOPP/ABVD in advanced stage Hodgkin’s disease
Regimens | No. of cases | Stage | FFP (%) | OS (%) |
|---|---|---|---|---|
114 | 63 | 64 | ||
MOPP × 3 → RT → MOPP × 3 | IIB, IIIA–B | |||
vs. ABVD × 3 → RT → ABVD × 3 | 118 | 81 | 71 | |
MOPP | 43 | 37 | 58 | |
vs. | IV A–B | |||
MOPP/ABVD | 45 | 61 | 69 | |
MOPP/ABVD alternating | 211 | I–IIB, IIAX, | 67 | 74 |
vs. | IIIA/B, | |||
MOPP/ABVD hybrid | 204 | IV A/B | 69 | 72 |
CALGB [134] | ||||
MOPP | 123 | 37 | 66 | |
vs. ABVD | 123 | IIIA2, IIIB, IVA/B | 52 | 73 |
vs. | ||||
MOPP/ABVD | 115 | 50 | 75 | |
Vancouver [84] | ||||
MOPP/ABV hybrid | 153 | IIIB, IVA, B | 71 | 81 |
vs. | ||||
MOPP/ABVD alternating | 148 | 67 | 83 | |
Intergroup [86] | ||||
MOPP/ABVD alternating vs. | 344 | IIIA2/B, IV A/B | 54 | 71 |
MOPP/ABV hybrid | 347 | 64 | 79 | |
New Intensive Combinations
Optimization of dose intensity, inclusion of new active drugs, and the appropriate use of hematopoietic growth factors have been studied as methods by which the cure rate might be enhanced in patients with advanced Hodgkin’s disease.
A complete remission rate of 94 % was reported with MOPPEBVCAD (mechlorethamine, vincristine, procarbazine, prednisone, epidoxorubicin, bleomycin, vinblastine, lomustine, melphalan, and vindesine) in 145 patients after six courses of the regimen followed by low-dose irradiation to sites of bulky disease at diagnosis and/or to sites incompletely responding to chemotherapy [92]. Tumor-specific, overall, relapse-free, and failure-free survivals at 5 years were 89, 86, 82, and 78 %, respectively. Most unfavorable prognostic factors lost their clinical significance, with the exception of age and lymphocyte-depletion histology. After a median follow-up of 66 months, seven patients developed second cancers, including three myelodysplasias. As this regimen includes a number of drugs with leukemogenic potential, a longer follow-up period with special attention to late sequelae is needed to determine the role, if any, this regimen may ultimately play in the treatment of advanced Hodgkin’s disease. Furthermore, many of the drugs used are today obsolete.
There has been much enthusiasm until recently for the BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) and escalated BEACOPP regimens developed by the German group. In an initial report [88], moderate dose escalation over the standard BEACOPP regimen was performed with granulocyte colony-stimulating factor (G-CSF) support, and maximal doses of three component drugs (doxorubicin, etoposide, cyclophosphamide) were given with acceptable hematological toxicity. The intended doses of doxorubicin, etoposide, and cyclophosphamide were substantially escalated from 25 to 35, from 650 to 1,200, and from 100 to 200 mg/m2, respectively. Of 60 patients enrolled, 56 (93 %) achieved complete remission, with an overall survival and freedom from progression at 32 months of 91 and 90 %, respectively. Another report gave the results of a randomized comparison of standard BEACOPP with escalated BEACOPP and COPP–ABVD, involving 505 evaluable patients with advanced-stage (IIB, IIIA with risk factors, IIIB, and IV) disease. Four courses of COPP–ABVD were compared with eight courses of baseline BEACOPP without G-CSF and with eight courses of escalated BEACOPP with G-CSF, all followed by radiotherapy to bulky sites (30 Gy) and to areas of residual disease (40 Gy) [88]. The interim analysis at 23 months showed a significant inferiority of the COPP-ABVD regimen in terms of progression rate and freedom from progression compared with the pooled results of both BEACOPP schemes. The 24-month freedom from progression rate was 75 % for COPP/ABVD and 84 % for BEACOPP pooled (p = 0.034). There were insufficient data to compare the two BEACOPP variants. The acute toxicity of COPP/ABVD and baseline BEACOPP was similar, whereas escalated BEACOPP showed increased but manageable hematological toxicity. Therefore, recruitment in the COPP/ABVD arm was discontinued. In a further interim analysis of 689 patients (COPP/ABVD 235 patients, baseline BEA COPP 241, escalated BEACOPP 213), escalated BEACOPP showed better freedom from progression at 2 years than baseline BEACOPP (89 versus 81 %). As yet, no significant differences in survival have emerged, however. Of concern is the higher number of secondary acute leukemias observed with escalated BEACOPP (four versus one) whereas secondary non-Hodgkin’s lymphomas (three cases) occurred only in the baseline BEACOPP group. Nevertheless, the authors concluded that results with escalated BEACOPP in advanced-stage Hodgkin’s lymphoma challenged ABVD as the standard of care for this population [135]. The same study group tested BEACOPP without etoposide (BACOPP) in elderly patients (age 60–75 years) with early-stage disease with unfavorable characteristics, or advanced-stage patients. The regimen was effective in this population but there was an unacceptably high toxic death rate of 11 % [136]. In an important presentation, Gianni et al. [137] demonstrated comparable 3-year overall survival with ABVD or BEACOPP first-line therapy for advanced-stage patients plus autologous transplant as salvage for relapse. Freedom from progression rate at 3 years was 16 % higher in the BEACOPP group, but the freedom from second progression rates were similar, as were overall survival rates. The authors concluded that 71 % of the ABVD-treated patients were cured by initial therapy and would have been overtreated if they had received BEACOPP instead. BEACOPP was associated with a fourfold higher toxic death rate compared with ABVD.
In the HD12 trial, the German Hodgkin’s Study Group attempted to determine whether eight cycles of escalated BEACOPP followed by irradiation might represent overtreatment. Advanced-stage Hodgkin’s lymphoma patients were randomized to receive four or eight cycles of escalated BEACOPP followed by four cycles of baseline BEACOPP. A second randomization assigned patients to receive radiotherapy as described in the previous trial or no radiotherapy. The study demonstrates that substantial reduction in irradiation dose after BEACOPP chemotherapy is warranted [138].
The Stanford group adjusted the Stanford V regimen, consisting of an abbreviated 12-week administration of the seven more active drugs, maintaining dose intensity of active agents but reducing the cumulative dose of doxorubicin, bleomycin, and mechlorethamine. In this trial, 47 patients were treated with the Stanford V regimen followed by radiotherapy (36 Gy) to lymph nodes 5 cm or larger in size and/or macroscopic splenic disease. The recently updated results based on a median follow-up period of 4.8 years show that 45 patients were alive and 40 had been continuously disease-free. The estimated freedom from progression and overall survival at 5 years were 85 and 96 %, respectively. One patient died from acute leukemia. Six of seven relapsed patients received high-dose therapy with autologous stem cell support and achieved freedom from second progression. As noted previously, a randomized comparison between Stanford V and ABVD found no difference in any outcome measure between the two [91].
Among numerous newer multidrug regimens in which etoposide was substituted for vincristine and epidoxorubicin for doxorubicin, it is worth mentioning VEBEP (etoposide, epidoxorubicin, bleomycin, cyclophosphamide, and prednisone). The Milan group reported their results with this regimen given every 21 days without growth factor support and followed by radiation therapy (30–36 Gy) to pretreatment-involved nodal sites. In 73 patients with stages IIB or III–IV or relapsing after radiotherapy, complete remission was achieved in 94 % of cases with freedom from progression and overall survival at 6 years of 78 and 82 %, respectively. Hematological toxicity was acceptable, with 86 % grade IV neutropenia, which resolved in the majority of cases by the next treatment cycle. The majority of male patients developed gonadal damage, reversible in half of them. No secondary leukemia or myelodysplasia had been detected at the time of publication [139]. Overall (Table 45.8), these regimens as well as others induce complete remission in up to 99 % of cases with freedom from progression at 5 years of about 80 %. None of these regimens are clearly superior to ABVD.
High-Dose Consolidation with Stem Cell Support
A number of nonrandomized trials have tested high-dose consolidation after achievement of complete remission with standard regimens in patients with particularly adverse prognostic presentations. Up to now there has been no evidence for the usefulness of such an approach in any subset of patients. As a matter of fact, up to 40 % of relapsed patients are cured with high-dose chemotherapy as salvage treatment (see discussion that follows). Thus, giving high-dose consolidation therapy may be overtreatment.
Carella et al. studied one course of CBV (cyclophosphamide, BCNU, etoposide) or BEAM (BCNU, etoposide, cytarabine, melphalan) followed by peripheral stem cell reinfusion in 22 patients with advanced-stage disease in first remission after MOPP-ABVD. Selection criteria consisted of bulky disease, age over 40 years, high serum lactate dehydrogenase (LDH) level, and anemia at onset. After a median follow-up time of 83 months, overall and RFS were 80 and 77 %, respectively [141].
Delain et al. [142] treated 26 high-risk patients with an estimated probability of cure less than 40 % (stage IV and at least two of the following factors: B symptoms, bulky mediastinal disease, two or more extranodal sites, bone marrow involvement, inguinal node involvement, high LDH serum level, or low hematocrit) with high-dose chemotherapy and peripheral blood stem cell support (PBSCT) as consolidation of first-line chemotherapy. At the time of transplantation, 19 patients were in complete remission and 7 in good partial remission after MOPP-ABVD or similar regimens. Actuarial 5-year overall survival, freedom from progression, and event-free survival were 69, 79, and 58 %, respectively. Only normal serum LDH level was found statistically significant as a prognostic indicator in univariate analysis. Of note, procedure-related mortality in the first 90 days after engraftment was 7 %. Better results were obtained by Nademanee in 20 high-risk patients according to the same criteria, who were given high-dose therapy and total-body irradiation after complete remission induction. All patients were alive and disease-free at 43 months [143]. Again, it must be stressed that all these results come from small nonrandomized heterogeneous series and must be evaluated with caution.
Role of Consolidation Radiotherapy in Advanced Stage
Soon after MOPP was introduced, it became clear that the majority of chemotherapy relapses occurred at sites of initial disease, particularly nodal sites and sites of bulky tumor, and that addition of radiotherapy markedly reduced the frequency of these recurrences, thus increasing the rates of both complete remission and disease-free survival [144, 145]. Because of these observations, adjuvant irradiation was widely adopted without demonstration of which, if any, Hodgkin’s lymphoma subsets benefited with improved overall survival. However, with growing awareness of the second tumor risk associated with irradiation, both radiotherapists and chemotherapists have reservations about this approach [146, 147].
There is general agreement on the need for combined-modality treatment in patients with mediastinal bulky disease [146–148]. Disease-free survival of 80–90 % is observed after combined-modality treatment, which is nearly twice that reported after MOPP or radiation alone. Otherwise, radiotherapy is not useful in patients suspected of residual disease after treatment. Some partial remissions can be converted to complete remissions, but such patients do not show an increased cure rate according to a number of randomized trials [148, 149].
The issue of whether irradiation has a role as consolidation therapy after complete remission is achieved is still a matter of debate. Groups at Yale, Duke, and Memorial Sloan-Kettering strongly support low-dose adjuvant irradiation (18–30 Gy) to previously involved sites for patients in remission after chemotherapy. In a cohort of 184 Yale patients with either newly diagnosed stage IIIB or IV disease or recurrent disease after irradiation alone, overall survival was 54 % at 15 years [150]. However, an unacceptable incidence of second neoplasms was observed in those treated with combined-modality therapy for disease recurrence after radiation alone (41 % at 20 years versus 12 % in the newly diagnosed patients) [112]. In a similar analysis at Memorial Sloan-Kettering, the actuarial 10-year overall survival and progression-free survival were 74 and 70 %, respectively [151]. Second-malignancy incidence was only 4 %, but the median duration of observation was too short to properly address that issue. A German investigation showed that while 20 Gy is sufficient to control initial sites of nonbulky disease or uninvolved sites following two double cycles of COPP/ABVD relapse patterns indicate that patients destined to relapse need more systemic rather than local therapy [152]. In a SWOG study, 278 patients in complete remission following a MOPP–ABVD-derived regimen were randomized to receive low-dose irradiation (10–20 Gy) to previously involved sites or no further treatment. The 5-year remission duration estimated at 79 % for patients who received radiation therapy was not significantly different from the 68 % observed in those who did not. Although low-dose irradiation improved the 5-year remission duration in the subgroups with nodular sclerosis (82 versus 60 %, p = 0.002) or bulky disease (75 versus 57 %, p = 0.05), overall 5-year survival was not improved in any subgroup [153]. The GELA group [154] published the results of a randomized comparison between two cycles of chemotherapy and nodal radiotherapy consolidation for patients with stage IIIB-IV Hodgkin’s disease in complete remission or good partial remission after six cycles of MOPP-ABV hybrid or ABVPP (doxorubicin, bleomycin, vinblastine, procarbazine, prednisone). After induction therapy, 418 patients were evaluable for consolidation treatment comparisons. After a median follow-up period of 48 months, the 5-year freedom from progression did not differ between chemotherapy (79 %), and combined modality (74 %). After MOPP-ABV, 5-year overall survival was similar for chemotherapy (85 %) and combined modality (88 %); after ABVPP the 5-year survival was 94 % for chemotherapy and 78 % for combined modality therapy. In conclusion, these results do not support the use of consolidation with radiotherapy instead of two further courses of chemotherapy after doxorubicin-induced complete remission for patients with advanced Hodgkin’s disease.
In 1998 [127], the International Database on Hodgkin’s Disease Overview Study Group published a meta-analysis of 1,740 patients from 14 controlled adjuvant irradiation clinical trials. In studies comparing the addition of radiation to chemotherapy versus the same chemotherapy alone, tumor control at 10 years was significantly improved by 11 %, but overall survival was similar. Trials in which the addition of radiation to chemotherapy was compared with the addition of further chemotherapy showed that there was no difference in tumor control in the two groups if an appropriate number of drug cycles were administered. However, overall survival was 8 % better in the chemotherapy alone group because of fewer late treatment-related deaths. Adjuvant radiotherapy was thus recommended only for a few specific indications such as bulky mediastinal disease. Thus, according to “evidence-based-medicine” criteria, there is no clear indication for consolidation therapy after complete remission in advanced stages of Hodgkin’s disease.
Salvage Therapy
The vast majority of relapsing patients will require some form of systemic therapy. The issues involved in salvage treatment of Hodgkin’s disease revolve around such concepts as non-cross-resistant chemotherapy, multidrug resistance, and response to escalated doses of chemotherapeutic agents [155, 156].
Prognostic Factors and Choice of Treatment
Salvage treatment for Hodgkin’s lymphoma is required for essentially four subsets of patients with different prognostic implications (1) patients relapsing after radiotherapy alone; (2) patients relapsing after more or (3) less than 12 months from the achievement of complete remission with first-line chemotherapy; and (4) chemoresistant induction failure patients. At present, the widespread use of high-dose chemotherapy with stem cell support in virtually all patients relapsing after chemotherapy has eliminated the distinction between categories (2) and (3) (see discussion that follows). As a matter of fact, the number of cases in the first group is progressively decreasing, as only a few patients are now given front-line radiotherapy alone (see earlier text).
In the past, duration of first complete remission has been considered the main prognostic factor predicting second chance of cure. In fact, a number of trials demonstrated prolonged disease-free survival by retreatment with the same regimen that resulted in a prolonged initial complete response. Of 32 patients relapsing after primary chemotherapy at the NCI, 59 % achieved a second complete remission when retreated with MOPP [41]. However, only 29 % of patients whose initial remission was less than 1 year achieved a second complete remission, compared with 93 % of patients whose initial complete remission was >1 year. The duration of second remission was also longer in patients whose initial complete remission exceeded 1 year than in those whose initial remission was less than 12 months. Similar results were also reported by the Milan group [157, 158], as well as by other investigators.
Today, the duration of first complete remission represents only one of the prognostic variables considered when second-line treatment is planned. A number of other factors, such as extension of disease at relapse, presence or absence of B symptoms, performance status, and response rate to induction therapy preceding high-dose consolidation, have demonstrated their prognostic significance in this setting [159].
Relapse After Radiation Therapy
As previously noted, 20–40 % of patients with stages I and II Hodgkin’s disease who are treated with primary radiation therapy will eventually relapse within 3 years. In a few instances, radiation therapy alone has been successfully used to salvage patients when the relapse occurred in a single lymph node chain more than 5 years after completion of the initial radiotherapy. In all other cases, the survival of patients treated with chemotherapy after radiation relapse is at least equal to that of advanced-stage patients initially treated with the same chemotherapy combination. Indeed, an apparent survival advantage of patients with radiation relapses over the primary treatment group has been attributed to a more favorable patient mix, with generally more limited extension of disease. Overall and disease-free survival range from 60 to 80 % [160, 161].
Even in this case, stage at relapse is an important prognostic variable. At Stanford the 10-year relapse-free survival was 88, 58, and 34 %, respectively, for those in stage IA, in stage IIA or IIIA, and in stage IV or with B symptoms at the time of relapse. As far as the choice of chemotherapy regimen is concerned, the same considerations outlined for first-line chemotherapy are of value. From the available evidence, ABVD exhibits the same superiority over other regimens for post-radiation recurrence that it does in initial treatment of advanced disease [80, 82].
Relapse After Chemotherapy
The treatment of choice for patients relapsing after chemotherapy has been historically represented by non-cross-resistant regimens at conventional doses. In the past, relapses after MOPP could be safely treated with ABVD with a second chance of cure as high as 40 % [162]. However, the vast majority of patients now receive front-line treatment with ABVD or some variant of it. Thus, a number of new regimens have been devised that induce complete remission in no more than 50 % of all patients, with 50–70 % of the responders eventually relapsing again. Such patients have a probability of survival at 5 years after relapse of only 15–30 % [163–165].
Somewhat better results come from regimens containing intermediate- or high-dose ifosfamide combined with etoposide or new active drugs such as vinorelbine [166–168]. In small series, up to 70 % of patients are disease-free after 2 years [166], and of note, there is no difference in outcome between relapsed and primary refractory cases. MVC mitoxantrone 8 mg/m2 on days 1–3, vinblastine 8 mg/m2 days 1 and 22, and lomustine 100 mg/m2 day1 is a new regimen given every 6–8 weeks for four cycles. A 91 % CR rate with a median duration of response of 11 months and a median overall survival rate of 34 % was reported in 45 relapsed patients most of whom received ABVD as initial treatment [140]. These results are excellent, but a prospective comparative trial is required before the true relative efficacy of that regimen is known.
High-dose therapy with autologous stem cell rescue (Tables 45.9 and 45.10) is now applicable to a large proportion of patients with relapsed Hodgkin’s lymphoma, as the use of growth factors and other supportive care strategies have definitively improved the safety of such an approach. The trend toward a better outcome for patients treated by high-dose therapy, along with the low mortality rate, now reported to be less than 1 % at most centers, may eliminate the need for a randomized comparison between high-dose and conventional therapy. Moreover, patients with relapsed disease are generally young in good general condition and represent ideal candidates for intensive procedures. On examining the literature (Table 45.9), a 5-year freedom from progression of approximately 40–50 % emerges despite the heterogeneity of case series in terms of the number of patients treated, induction and conditioning regimens, and eligibility criteria [98, 159, 169–173, 176, 179].
Table 45.9
High-dose therapy with stem-cell rescue for patients in first relapse
Author (ref) | N | Selection criteria | Conditioning regimen | FFP (%) | FU (years) |
|---|---|---|---|---|---|
Chopra [169] | 52 | Relapse within 1 year | BEAM | 47 | 5 |
At least 2 previous regimens | |||||
Nademanee [143] | 43 | Relapse within 1 year | BCNU/VP16/CTX | 40 | 3 |
Non-CR after salvage therapy | TBI/VP16/CTX | ||||
Bierman [170] | 58 | Relapse after chemotherapy | CBV | 40 | 5 |
Reece [171] | 58 | Relapse after chemotherapy | CBV ± CDDP | 61 | 5 |
Yuen [160] | 47 | Relapse after chemotherapy | BCNU/VP16/CTX | 56/50 | 4 |
1 year, more or less | TBI/VP16/CTX | ||||
Wheeler [172] | 42 | Relapse after CR | CBV | 44 | 4 |
aSweethenham [173] | 139 | Relapse after CR | BEAM/CBV/others ± TBI | 44.7 | 5 |
bBrice [174] | 220 | Relapse after CR | CBV/BEAM/BEAC/others | 71 | 5 |