Acute haemorrhage
Malignant disease (e.g. carcinoma, Hodgkin and non-Hodgkin lymphoma)
Chronic inflammatory disorders (e.g. rheumatoid arthritis, ulcerative colitis)
Acute inflammation
Post-operation
Splenectomy and hyposplenism
Marrow recovery from drug suppression, or response to haematinic following deficiency
Iron deficiency
Drug therapy (e.g. corticosteroids, adrenaline)
Table 12.2
Risk stratification in ET
High-risk |
Age >60 and/or high-risk feature(s) Platelet count >1,500 × 109/L (current or previous) History of ischaemia, thrombosis or embolic events Haemorrhage considered secondary to ET Hypertension or diabetes mellitus |
Intermediate-risk |
Age 40–59 years |
No high-risk features |
Low-risk |
Age less than 40 years |
No high-risk features |
The presence of mutations in the JAK2 and MPL genes has now enabled the positive diagnosis of ET. However, while JAK2 V617F testing is widely used as an initial screening test, MPL analysis should generally be reserved for those cases negative for the JAK2 mutation. Patients who are wild type for JAK2 and MPL should be examined carefully to exclude a secondary or reactive thrombocytosis, most commonly the result of inflammatory and infectious disorders, iron deficiency and haemorrhage, malignancy and other types of haematopoietic neoplasms (Table 12.2).
Importantly, the presence of a BCR–ABL1 fusion gene excludes the diagnosis of ET in those rare cases of chronic myeloid leukaemia that present with an isolated thrombocytosis. A few patients with a myelodysplastic syndrome (MDS) present with thrombocytosis, although in such cases the megakaryocytic morphology, with small hypolobulated forms, is quite distinct from the changes seen in ET. Furthermore, a third to one half of patients with the 5q- syndrome exhibit thrombocytosis, although this disorder is invariably characterised by a macrocytic anaemia. Finally, an entity termed refractory anaemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T) has been recognised, a disorder that exhibits both myelodysplastic (RARS-like) and myeloproliferative (ET-like) features. Such a MDS/MPN disorder is thought to develop from a pre-existing RARS through acquisition of somatic mutations of JAK2, MPL or other as-yet-unknown genes [7].
Treatment of Essential Thrombocythemia
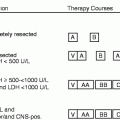
Since thrombotic events are the predominant complication of ET, all patients should be screened for known predisposing factors, including systemic hypertension, hyperlipidaemia and diabetes mellitus, while a smoking history should be obtained and any cardiovascular risk factors aggressively managed. The impact of other potential thrombotic risk factors, including acquired thrombophilia, the presence of either JAK2 V617F or MPL mutations, an abnormal MPL expression or the presence of a leucocytosis, remains controversial. All patients, however, should be stratified according to their clinical risk of thrombotic and/or haemorrhagic complications, since this greatly influences the therapeutic approach. The most widely accepted risk stratification separates patients into high-, intermediate and low-risk cases (Table 12.3).
Table 12.3
WHO criteria for essential thrombocythemia (ET)
Sustained platelet count ≥450 × 109/L |
Bone marrow biopsy specimen showing proliferation mainly of megakaryocytes with increased numbers of enlarged, mature megakaryocytes. No significant increase or left shift of neutrophil granulopoiesis or erythropoiesis |
Not meeting WHO criteria for polycythemia vera, primary myelofibrosis, BCR–ABL1-positive chronic myelogenous leukaemia or myelodysplastic syndrome or other myeloid neoplasm |
Demonstration of JAK2 V617F or other clonal marker, or in the absence of JAK2 V617F, no evidence for reactive thrombocytosis |
High-Risk Disease
High-risk individuals include those over the age of 60 years, those who have had a previous ET-related thrombotic or haemorrhagic event, patients who require therapy for systemic hypertension or diabetes or those with a platelet count greater than 1,500 × 109/L. It should be noted, however, that microvascular symptoms are not generally regarded as thrombotic events for the purpose of risk assessment, unless they are severe, or fail to respond to platelet anti-aggregating agents. It is now accepted that high-risk patients should be treated with platelet lowering agents, ideally to a target value of 400 × 109/L, together with aspirin. Such a conclusion is supported by an Italian prospective study that randomised 114 such cases to receive either hydroxycarbamide or no cytoreductive therapy. After a median follow-up of 27 months, patients on hydroxycarbamide suffered significantly fewer thrombotic events [8].
Which therapy should be offered as first-line? This question has been answered by the Medical Research Council’s Primary Thrombocythemia-1 (MRC PT-1) trial, the largest prospective randomised trial ever undertaken in the field of myeloproliferative neoplasms [9]. In this study, 809 high-risk patients with ET were randomised to receive hydroxycarbamide plus aspirin, or anagrelide plus aspirin. Compared to hydroxycarbamide plus aspirin, patients in the anagrelide arm suffered higher rates of arterial thrombosis, major haemorrhage and myelofibrotic transformation, but intriguingly a reduced rate of venous thromboembolism. These data support the view that hydroxycarbamide and aspirin should be prescribed as first-line therapy for the majority of high-risk ET cases. Importantly, a comparison of arterial thrombotic rates in the PT-1 anagrelide arm (8 % at 2 years) with the untreated arm of the Cortelazzo study (26 % at 2 years) suggests that anagrelide provides partial protection from this complication. The PT1 study, therefore, supports anagrelide’s licensed indication as second-line therapy for patients refractory, or intolerant, of hydroxycarbamide (Table 12.4).
Table 12.4
Consensus definition of clinical resistance or intolerance to hydroxycarbamide in patients with ET
Platelet count of | >600,000/μL after 3 months of treatment with at least 2 g/day hydroxycarbamide (2.5 g/day in patients with a body mass of >80 kg) >400,000/μL and leucocytes <2,500/μL at any dose of hydroxycarbamide >400,000/μL and haemoglobin <10 g/dL at any dose of hydroxycarbamide |
Presence of leg ulcers or other unacceptable mucocutaneous manifestations at any dose of hydroxycarbamide | |
Fever related to hydroxycarbamide | |
Low- and Intermediate Risk Disease
Patients under the age of 40 years, with no high-risk features, are regarded as low risk and should be offered anti-platelet drugs only, since such cases have a thrombotic risk that is only slightly greater than, or equal to, that in the normal population. It will be important, however, to gain better quality information about this patient group, ideally in large clinical studies, such as the ongoing low-risk arm of the UK PT1 study. The management of intermediate risk cases, defined as those aged 40–60 years with no high-risk features, remains unclear and routine cytoreductive therapy should be avoided until the results of an international randomised study (PT-1) are known.
Platelet Anti-Aggregating Agents
Microvascular occlusion in ET, resulting in symptoms such as painful digits, that can lead to gangrene, transient ischaemic attacks, migraine and erythromyelalgia is improved by aspirin therapy [10]. However, the only randomised controlled trial investigating the role of aspirin in MPN has been in the related disorder polycythemia vera (PV). The so-called ECLAP study (European Collaboration on Low-dose Aspirin in PV) nevertheless supports the safety and utility of routinely prescribed aspirin, as treated patients had a significantly reduced risk of thrombotic complications, including non-fatal myocardial infarction and stroke, death from cardiovascular causes, pulmonary embolism and major venous thrombosis [11]. A maintenance daily dose of 75 mg is usually sufficient and reduces the risk of peptic ulceration associated with larger doses. Treatment should be avoided in patients with a history of bleeding, or in those with high platelet counts, i.e. 1,000–1,500 × 109/L, especially in the context of a bone marrow trephine [12]. For the occasional patient with ongoing thrombotic events, despite adequate platelet control, consideration should be given to the combination of aspirin and clopidogrel, while clopidogrel alone may be helpful in patients with aspirin-related side effects.
Hydroxycarbamide
Hydroxycarbamide, or hydroxyurea, is an anti-metabolite that slows DNA synthesis and repair by inhibiting ribonucleotide diphosphate reductase activity, an enzyme that catalyses the reduction of ribonucleotides. The end result is a block in cell cycle at the G1/S phase and cell death. Side effects are uncommon, mostly mild and reversible, and include neutropenia and anaemia, gastrointestinal tract symptoms (anorexia, nausea, vomiting and diarrhoea) and fever. Rare complications include pneumonitis, azoospermia and liver dysfunction. Prolonged use is associated with a range of dermatological complications, including actinic keratosis, squamous cell carcinoma and nail changes such as onychodystrophy and melanonychia. The evidence for teratogenicity is weak, although it is recommended that patients should avoid conception while on therapy [13].
Anagrelide
Anagrelide is an orally active imidazoquinazoline compound, with potent platelet-reducing activity as a result of inhibiting megakaryocyte size, ploidy and maturation, and consequently is not thought to be leukaemogenic. Its efficacy is well established in ET patients, including both treatment-naïve cases and those refractory to other cytoreductive agents, being effective in over 90 % of cases. The response is rapid, with most patients reaching the treatment target within a few weeks. The results of the largest randomised study, comparing hydroxycarbamide and anagrelide (MRC PT-1 Study), indicated that despite an equivalent platelet count, anagrelide-treated patients experience higher rates of arterial thrombosis, major haemorrhage and myelofibrotic transformation, while venous thrombosis was greater in the hydroxycarbamide arm [9]. In contrast, the recently published ANAHYDRET study suggests that anagrelide is not inferior to hydroxycarbamide [14]. However, it should be noted that the number of patients and end-point events were considerably less than that in the PT-1 study and, as a result, the ANAHYDRET study may not have been powered to detect a clinical difference. Overall, these two randomised studies support the use of anagrelide as second-line therapy in high-risk ET.
The most frequent side effects of anagrelide relate to its inhibition of cyclic nucleotide phosphoesterase III, an enzyme expressed in heart tissue and vascular smooth muscle, and include headache, palpitations and tachycardia. Such problems are usually mild and subside over time, with lower incidences reported during long-term therapy compared with the initial treatment phase. Side effects can be minimised by starting at a low dose (0.5 − 1.0 mg/day) and increasing gradually, while avoiding obvious precipitating factors, such as stress and anxiety, alcohol, caffeine and smoking. Benign palpitations that do not subside can be treated with low-dose beta-blockers (e.g. metoprolol or atenolol), especially if exercise or stress related. Aggravation of cardiac insufficiency is a rare but important side effect, and special caution is advised in patients with previous cardiac failure. Anagrelide may also be associated with a mild fall in haemoglobin levels as a result of its vasodilatory properties.
Alpha Interferon
The attraction of interferon, a presumed non-leukaemic and non-teratogenic agent with the potential to eradicate an abnormal clone, has led to the use of recombinant interferon-alpha (IFN-alpha), particularly in younger patients and during pregnancy. Early studies in ET showed that IFN-alpha can result in a reduction in platelet count to less than 600 × 109/L within 3 months, using an average of three million IU per day [15]. However, the use of conventional IFN has been limited by toxicity, leading to treatment discontinuation in approximately a third of patients. Side effects include flu-like symptoms, fatigue, musculoskeletal pain, depression and autoimmune disorders. Recently, pegylated interferon has been shown to be better tolerated, with only 8 % of patients with PV having to stop therapy at 1 year [16], a rate comparable with that reported for hydroxycarbamide. Furthermore, pegylated interferon alfa-2a (PEG-IFN-alpha-2a) has been shown to reduce the mutant allele burden in JAK2 V617F-positive ET patients, with 6 % of cases achieving a complete molecular remission [17]. Additional studies are required to confirm these interesting findings and to determine whether molecular responses are associated with improved clinical outcomes.
Busulphan
Suspicion of the potential long-term side effects of all alkylating agents, as well as the risk of prolonged bone marrow suppression, has limited the use of busulphan as a routine treatment of ET. Furthermore, its use has been associated with pulmonary toxicity, especially broncho-pulmonary dysplasia and pulmonary fibrosis, while a study reporting an increased risk of second malignancies after the sequential use of busulphan and hydroxycarbamide is a further cause for concern [18]. Although it has been successfully and conveniently used in small intermittent doses by some centres [19], its use should be confined to elderly patients. A relatively non-toxic but effective regimen consists of busulphan 2–4 mg/day for 1–2 weeks administered every 4–6 weeks until the platelet count is below 400 × 109/L, with further doses being prescribed once the count rises above this level.
Radio-Labelled Phosphorus
Radioactive phosphorus (32-P) has been used for the treatment of MPN for nearly 80 years. The isotope has a half-life of 14.3 days, is a pure beta emitter and has a maximum range in vivo of only 8 mm. It is an effective agent at controlling blood counts, has few acute side effects and rarely results in haematological complications [20]. A single intravenous dose of between 2.5 and 3.0 mCi/m2 is often sufficient to provide adequate control for up to 6–36 months, although occasionally a second smaller dose is required after 3–4 months. There is conclusive evidence, however, that it is leukaemogenic and as a result, its use should be restricted for patients over 70 years of age.
Essential Thrombocythemia in Childhood
It is essential to rigorously exclude secondary, or reactive, causes of thrombocytosis in paediatric practice, especially in young children. The rationale for this approach is that the annual incidence of newly diagnosed cases of ET in childhood is extremely rare (approximately one per ten million), i.e. 60 times lower than that for adults, and occurs at a median age of 11 years. Furthermore, thrombocytosis is a far more common finding in children who are sick, infected or post-surgical than in adults. Familial thrombocytosis, caused by mutations in TPO or TPO receptor (MPL) genes, should also be excluded [21]. These mutations, resulting in an over-expression of TPO, sustained intracellular signalling or a dysregulation of circulating TPO, account for 40–50 % of all children with primary thrombocytosis. The diagnostic criteria proposed for adults can still be applied to children, although interestingly, most paediatric cases appear polyclonal and lack JAK2 mutations.
Established cases of childhood ET are usually managed according to the recommendations highlighted earlier. However, there is very little safety or efficacy data regarding cytoreductive therapy in children and while hydroxycarbamide is usually used to initially control counts, an early switch to IFN-α or anagrelide should be encouraged for those requiring long-term treatment [22]. Aspirin, in contrast to adult practice, should be used with caution in children, due to the risks of Reye’s syndrome. If suspected, ammonia levels should be checked and aspirin should be stopped.
Essential Thrombocythemia in Pregnancy
Pregnancy is often associated with a spontaneous fall in the platelet count, sometimes to normal levels. Nevertheless, there is an increased first trimester loss in between 26 % and 36 % of ET pregnancies, compared to the anticipated loss in ‘normal’ pregnancies of only 15–20 %. In addition, there is a higher risk of intrauterine death, growth retardation and premature delivery (presumably associated with platelet vascular occlusion) so that the live birth rate in ET patients may only be a little over 50 %. Recent studies suggest that the presence of JAK2 V617F may increase the risk of pregnancy loss, although this association is not sufficiently strong to warrant an alteration in therapeutic management. In contrast to the impact on the foetus, maternal complications are relatively uncommon and no fatal events have been reported. Those thrombotic events that do occur tend to be minor, with most occurring in the postpartum period, a fact that emphasises the need for postpartum prophylaxis.
The management of ET in pregnancy is dependent on the patient’s disease risk and previous obstetric history. Maternal or foetal complications are more likely to occur in high-risk pregnancies, defined as the presence of any one of the following: previous maternal venous or arterial thrombosis, previous haemorrhage secondary to ET, previous complications in pregnancy caused by ET (unexplained three first trimester losses, intrauterine growth retardation, intrauterine death or still birth, severe pre-eclampsia, placental abruption or significant ante- or postpartum haemorrhage) and platelet count persistently greater than 1,500 × 109/L. Such high-risk pregnancies should be considered for cytoreductive therapy with IFN-α and low molecular weight heparin (reviewed 13). There have been no reported teratogenic side effects from IFN-α, although it is probably best avoided in patients with reduced fertility as this may be aggravated. First- and second-line cytoreductive agents, namely hydroxycarbamide and anagrelide, are contraindicated in pregnancy and ideally both drugs should be gradually withdrawn, 3–6 months prior to conception, and replaced by IFN-α. Low-dose aspirin appears safe and in the absence of any contraindications should be prescribed to all patients throughout pregnancy.
Those patients who have suffered a previous venous or arterial thrombosis should be offered thromboprophylaxis throughout pregnancy, with low molecular weight heparin. A recommended regime involves the administration of subcutaneous enoxaparin 40 mg daily, increasing to twice daily from 16 weeks and reducing to 40 mg/day for 6 weeks post-partum. In addition, patients should be encouraged to wear graded elastic compression stockings (GECS) throughout pregnancy and for 6–12 week post-partum. Breast feeding is safe with heparin and interferon and should be encouraged.
Surgery
A recent study has estimated that the risk of DVT after major surgery in patients with ET is at least fivefold increased [23], despite effective control of platelet count and the administration of standard anti-thrombotic prophylaxis. In addition, arterial thromboses are increased at around 3.8 % post-operatively, while there is a 10.5 % bleeding risk following surgery, a fact likely to be related to primary platelet dysfunction and the use of anti-platelet agents and anticoagulant therapy. Post-operative thromboprophylaxis with low molecular weight heparin is recommended, although prolonged use is unnecessary as the risk of venous and arterial thromboses dramatically reduces within 1 month of surgery. Anti-platelet drugs may be the optimal choice for ET patients at high risk of arterial thrombosis, although this approach should be balanced against the surgery-specific bleeding risk. It should be stressed, however, that prospective studies are needed to define the best anti-thrombotic management of this difficult group of patients [23].
Primary Myelofibrosis
PMF is a chronic stem cell disorder characterised by bone marrow fibrosis, extramedullary haematopoiesis, splenomegaly and a leuco-erythroblastic blood picture. It is an uncommon disorder, with a reported annual incidence ranging from 0.5 to 1.3 per 100,000 and although its aetiology is unknown, environmental factors may be relevant as the disorder has been linked in a small number of patients to radiation and benzene exposure. Recently, considerable progress has been made in understanding its pathogenesis, although this has yet to result in significant therapeutic advances. Indeed, its prognosis remains poor when compared to that of other BCR–ABL-negative MPN, with death resulting from cardiac failure, infection, haemorrhage and leukaemic transformation.
Diagnosis
The diagnostic criteria for PMF have recently been defined by the WHO and rely heavily on the features of the bone marrow trephine (Table 12.5). The differential diagnosis encompasses the many causes of bone marrow fibrosis (Table 12.6). Whereas increased fibrosis may be found in PV, ET and chronic myeloid leukaemia, myelofibrotic transformation of these conditions can occur with features resembling PMF.
Table 12.5
WHO diagnostic criteria for primary myelofibrosis: diagnosis requires meeting all three major and two minor criteria
Major criteria |
Presence of megakaryocyte proliferation and atypiaa, usually accompanied by either reticulin and/or collagen fibrosis or in the absence of significant reticulin fibrosis, the megakaryocyte changes must be accompanied by an increased bone marrow cellularity characterised by granulocytic proliferation and often decreased erythropoiesis (i.e. prefibrotic cellular phase disease). |
Not meeting the WHO criteria for polycythemia verab, BCR–ABL1+ chronic myelogenous leukaemiac, myelodysplastic syndromed or other myeloid neoplasms |
Demonstration of JAK2 V617F or other clonal marker (e.g. MPL W515K/L) or in the absence of a clonal marker, no evidence that the bone marrow fibrosis or other changes are secondary to infection, autoimmune disorder or other chronic inflammatory condition, hairy cell leukaemia or other lymphoid neoplasm, metastatic malignancy or toxic (chronic) myelopathiese |
Minor criteria |
Leucoerythroblastosis |
Increase in serum lactate dehydrogenase levelf |
Anaemiaf |
Splenomegalyf |
Table 12.6
Conditions associated with bone marrow fibrosis
Malignant | Non-malignant |
|---|---|
Primary myelofibrosis | Infections (e.g. TB, visceral leishmaniasis histoplasmosis, HIV) |
Other myeloprolifertaive neoplasms (e.g. PV, CML, ET) | Renal osteodystrophy |
Acute megakaryoblastic leukaemia (acute myelofibrosis) | Vitamin D deficiency |
Myelodysplastic syndrome | Hypothyroidism |
Acute myeloid leukaemia | Hyperthyroidism |
Acute lymphoblastic leukaemia | Gray platelet syndrome |
Hairy cell leukaemia | Systemic lupus erythematosus |
Hodgkin lymphoma | Scleroderma |
Other lymphoid neoplasms | Radiation exposure |
Multiple myeloma | Benzene exposure |
Systemic mastocytosis | Gaucher’s disease |
Metastatic carcinoma (e.g. breast, prostate, stomach) | Osteopetrosis |
Pathogenesis
Current evidence indicates that PMF is a true clonal disorder of the haematopoietic stem cell. Such a conclusion was first reached by the analysis of glucose-6-phosphate dehydrogenase (G-6PD) isoenzymes and subsequently by studies using X-linked restriction fragment length polymorphisms (RLFPs), which confirmed PMF to be characterized by monoclonal haematopoiesis. This conclusion is supported by the karyotype-based demonstration of clonality of erythroid, granulocyte–monocyte and granulocyte–monocyte–erythroid progenitors, but not fibroblasts [24]. Recurrent cytogenetic abnormalities occur in approximately 50 % of PMF cases at diagnosis, with the most common lesions, representing over 80 % of abnormal cases, being del(13)(q12;q22), del(20)(q11;q13), trisomy 8, trisomy 9, del(12)(p11;p13), monosomy or long arm deletion of chromosome 7 and partial trisomy 1q [25, 26]. No cytogenetic lesion appears specific for PMF, with the possible exception of der(6)t(1;6)(q23–25;p21–22), which has recently been proposed as a marker for PMF [27]. Interestingly, the gene coding the FK-506-binding protein 5 (FKBP51) is located in the involved region (6p21) and the protein has been shown to be over-expressed in CD34+ cell-derived megakaryocytes from PMF patients.
A major advance in the understanding of the disease’s pathogenesis was the identification of the somatic mutation JAK2 V617F, which occurs in approximately 50 % of cases. JAK2 V617F, a G to T substitution that generates valine instead of phenylalanine at position 617 within the JAK2 pseudokinase domain, leads to activation of multiple downstream signalling pathways, enabling cytokine-independent haematopoietic cell growth [28]. Somatic mutations in the thrombopoietin receptor, MPL, were subsequently documented in 5 % of JAK2 V617F-negative cases, confirming the importance of constitutive activation of the JAK-STAT pathway in the disease’s pathogenesis [29]. Although the discovery of JAK2 and MPL mutations has provided important insights, there remain a number of unanswered questions: for example how do the mutations lead to constitutive activation of the protein, what mutations are involved in those cases lacking a known genetic abnormality and what role does gene dosage play?
The pathogenesis of excessive bone marrow fibrosis that is a feature of PMF is believed to be a reaction to the underlying clonal haematopoietic tissue. The prevailing hypothesis involves an aberrant release of cytokines from the clonal megakaryocytes and monocytes that include transforming growth factor-β, basic fibroblast growth factor, epidermal growth factor, platelet-derived growth factor, vascular endothelial growth factor and calmodulin, all of which induce a secondary fibroblast proliferation and dysregulation of extracellular matrix formation [24]. The latter is composed of an excessive deposition of interstitial and basement membrane glycoproteins, including collagens type I, III, IV, V and VI, fibronectin, vitronectin, laminin and tenascin, as well as a marked increase in angiogenesis. Furthermore, the pathological ingestion of neutrophils by megakaryocytes (emperipolesis), induced by altered P-selectin expression, may contribute to cytokine release [30], while neutrophil-derived enzymes facilitate the release of CD34+ stem cells into the peripheral blood, a characteristic feature of PMF [31]. Interestingly, PMF CD34+ cells generate a 24-fold greater number of megakaryocytes (MKs) than normal CD34+ cells. Such derived MKs exhibit a delayed pattern of apoptosis and an over-expression of the anti-apoptotic BCL-xL protein, findings that lend support to the current hypothesis of the pathogenesis of PMF [32].
Clinical Features
PMF characteristically occurs after middle age, with a median age at presentation of 60 years. About a quarter of patients are asymptomatic at diagnosis and are identified following routine examination. The most common symptoms are the consequence of anaemia, namely fatigue, weakness, palpitations and dyspnoea. Palpable splenomegaly is characteristic and when massive can manifest in a range of complaints, particularly abdominal discomfort and early satiety. Splenic infarction, resulting from the organ outgrowing its blood supply, may produce transient discomfort, although rarely can result in severe abdominal pain simulating an abdominal emergency. Hepatomegaly occurs in approximately 70 % of cases and portal hypertension may result from increased hepatic blood flow or intra-hepatic obstruction. Constitutional symptoms may dominate the clinical picture, including low-grade fever, night sweats and weight loss, and their presence carries a poor prognosis [33]. Patients may also complain of bone pain, especially in the lower extremities. Bleeding may complicate the clinical course and, although often mild manifesting as petechiae and ecchymoses, can be life threatening due to massive gastrointestinal haemorrhage. The haemorrhagic diathesis may result from a combination of thrombocytopenia, acquired platelet dysfunction and low-grade disseminated intravascular coagulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree