Aldosterone-producing adenoma (APA)—30% of cases
Bilateral idiopathic hyperplasia (IHA)—60% of cases
Primary (unilateral) adrenal hyperplasia—2% of cases
Aldosterone-producing adrenocortical carcinoma—<1% of cases
Familial hyperaldosteronism (FH)
Glucocorticoid-remediable aldosteronism (FH type 1)—<1% of cases
FH type 2 (APA or IHA)—<6% of cases
FH type 3 (germline KCNJ5 mutations)—<1% of cases
FH type 4 (germline CACNA1H mutations)—<1% of cases
Ectopic aldosterone-producing adenoma or carcinoma—<0.1% of cases
Prevalence
In the past, clinicians would not consider the diagnosis of PA unless the patient presented with spontaneous hypokalemia, and then the diagnostic evaluation would require discontinuation of antihypertensive medications for at least 2 weeks. This diagnostic approach resulte d in predicted prevalence rates of <0.5% of hypertensive patients [3–9]. However, it is now recognized that most patients with PA are not hypokalemic [10–13] and that case detection testing can be completed while the patient is taking antihypertensive drugs with a simple blood test that yields the ratio of plasma aldosterone concentration (PAC) to PRA [11]. The use of the PAC/PRA ratio as a case detection test, followed by aldosterone suppression for confirmatory testing, has resulted in much higher prevalence estimates for PA—5–10% of all patients with hypertension [11–14].
Clinical Presentation
The diagnosis of PA is usually made in patients who are in the third to sixth decade of life. Few symptoms are specific to the syndrome. Patients with marked hypokalemia may have muscle weakness and cramping, headaches, palpitations, polydipsia, polyuria, nocturia, or a combination of these [10]. Periodic paralysis is a very rare presentation in Caucasians, but it is not an infrequent presentation in patients of Asian descent [15]. Polyuria and nocturia are a result of hypokalemia-induced renal concentrating defect, and the presentation is frequently mistaken for prostatism in men. There are no specific physical findings. Edema is not a common finding because of the phenomenon of mineralocorticoid escape. The degree of hypertension is typically moderate to severe and may be resistant to usual pharmacologic treatments [10, 16]. In the first 262 cases of PA diagnosed at Mayo Clinic (1957–1986), the highest blood pressure was 260/155 mmHg; the mean (±SD) was 184/112 ± 28/16 mmHg [16]. Patients with APA tend to have higher blood pressures than those with IHA. Hypokalemia is frequently absent, so all patients with hypertension are candidates for this disorder. In other patients, the hypokalemia becomes evident only with the addition of a potassium-wasting diuretic. Deep-seated renal cysts are found in up to 60% of patients with PA and chronic hypokalemia [17]. Because of a reset osmostat, the serum sodium concentration tends to be high-normal or slightly above the upper limit of normal. This clinical clue is very useful in the initial assessment for potential PA.
Several studies have shown that patients with PA are at higher risk than other patients with hypertension for target organ damage of the heart and kidney [18, 19]. Chronic kidney disease is common in patients with long-standing PA [20]. When matched for age, blood pressure, and duration of hypertension, patients with PA have greater left ventricular mass measurements than patients with other types of hypertension (e.g., pheochromocytoma, Cushing syndrome, essential hypertension) [21]. In patients with APA, the left ventricular wall thickness and mass were markedly decreased 1 year afte r adrenalectomy [22]. A case-control study of 124 patients with PA and 465 patients with essential hypertension (matched for age, sex, and systolic and diastolic blood pressure) found that patients presenting with either APA or IHA had a significantly higher rate of cardiovascular events (e.g., stroke, atrial fibrillation, myocardial infarction) than the matched patients with essential hypertension [19]. A negative effect of circulating aldosterone on cardiac function was found in young nonhypertensive subjects with GRA who had increased left ventricular wall thickness and reduced diastolic function compared with age- and sex-matched controls [18].
Diagnosis
The diagnostic approach to PA can be considered in three phases: case detection tests, confirmatory tests, and subtype evaluation tests.
Case Detection Tests
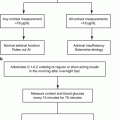
Spontaneous hypokalemia is uncommon in patients with uncomplicated hypertension; when present, it strongly suggests associated mineralocorticoid excess. However, several studies have shown that most patients with PA have baseline serum levels of potassium in the normal range [11, 13]. Therefore, hypokalemia should not be the major criterion used to trigger case detection testing for PA. Patients with hypertension and hypokalemia (regardless of presumed cause), treatment-resistant hypertension (poor control on three antihypertensive drugs), severe hypertension (≥150 mmHg systolic or ≥100 mmHg diastolic), hypertension and an incidental adrenal mass, or onset of hypertension at a young age should undergo case detection testing for PA (Fig. 11.1) [10, 11].
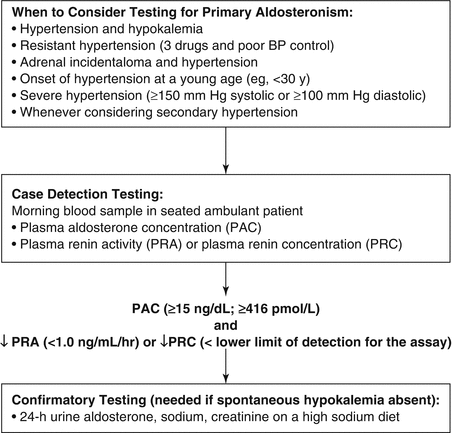

Fig. 11.1
Algorithm provides guidance on when to consider testing for PA, use of the plasma aldosterone concentration (PAC) and plasma renin activity (PRA) as a case detection tool, and 24-hour urinary aldosterone excretion for confirmatory testing. PRC plasma renin concentration
In patients with suspected PA, screening can be accomplished by paired measurements of PAC and PRA in a random morning ambulatory blood sample (preferably obtained between 8 and 10 a.m.). This test may be performed while the patient is taking antihypertensive medications (with some exceptions, discussed later) and without posture stimulation [10]. Hypokalemia reduces the secretion of aldosterone, and it is optimal to restore the serum level of potassium to near-normal before performing diagnostic studies.
It may be difficult to interpret data obtained from patients treated with a mineralocorticoid receptor antagonist (spironolactone and eplerenone). These drugs prevent aldosterone from activating the receptor, resulting sequentially in sodium loss, a decrease in plasma volume, and an elevation in PRA, which will reduce the utility of the PAC/PRA ratio. For this reason, spironolactone and eplerenone should not be initiated until the evaluation is completed and the final decisions about treatment are made. However, there are rare exceptions to this rule. For example, if the patient is hypokalemic despite treatment with spironolactone or eplerenone, then the mineralocorticoid receptors are not fully blocked, and PRA or PRC should be suppressed in such a patient with PA. In this unique circumstance, the evaluation for PA can proceed despite treatment with mineralocorticoid receptor antagonists. However, in most patients already receiving spironolactone, therapy should be discontinued for at least 6 weeks. Other potassium-sparing diuretics, such as amiloride and triamterene, usually do not interfere with testing unless the patient is on high doses.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have the potential to falsely elevate the PRA in a patient with PA. Therefore, the finding of a detectable PRA level or a low PAC/PRA ratio in a patient taking one of these drugs does not exclude the diagnosis of PA. However, an undetectably low PRA level in a patient taking an ACE inhibitor or ARB makes PA likely.
The PAC/PRA ratio, first proposed as a case detection test for PA in 1981 [23], is based on the concept of paired hormone measurements. The PAC is measured in nanograms per deciliter (ng/dL) and the PRA in nanograms per milliliter per hour (ng/mL/h). In a hypertensive hypokalemic patient, secondary hyperaldosteronism should be considered if both PRA and PAC are increased and the PAC/PRA ratio is <10 (e.g., renovascular disease). An alternative source of mineralocorticoid receptor agonism should be considered if both PRA and PAC are suppressed (e.g., hypercortisolism). PA should be suspected if the PRA is suppressed (<1.0 ng/mL/h) and the PAC is increased (e.g., >15 ng/dL). Although there is some uncertainty about test characteristics and lack of standardization, the PAC/PRA ratio is widely accepted as the case detection test of choice for PA [11, 24].
It is important to understand that the lower limit of detection varies among different PRA assays and can have a dramatic effect on the PAC/PRA ratio. As an example, if the lower limit of detection for PRA is 0.6 ng/mL/h and the PAC is 16 ng/dL, then the PAC/PRA ratio with an “undetectable” PRA would be 27; however, if the lower limit of detection for PRA is 0.1 ng/mL/h, the same PAC level would yield a PAC/PRA ratio of 160. Thus, the cutoff for a “high” PAC/PRA ratio is laboratory dependent and, more specifically, PRA assay dependent. In a retrospective study, the combination of a PAC/PRA ratio >30 and a PAC level >20 ng/dL had a sensitivity of 90% and a specificity of 91% for APA [25]. At Mayo Clinic, the combination of a PAC/PRA ratio of 20 or higher and a PAC level of at least 15 ng/dL is found in more than 90% of patients with surgically confirmed APA [16]. In patients without PA, most of the variation occurs within the normal range [26]. A high PAC/PRA ratio is a positive case detection test, a finding that warrants further testing [11].
It is critical for the clinician to recognize that the PAC/PRA ratio is only a case detection tool, and, in the absence of spontaneous hypokalemia, all positive results should be followed by a confirmatory aldosterone suppression test to verify autonomous aldosterone production before treatment is initiated [11]. In a study of 118 subjects with essential hypertension, neither antihypertensive medications nor acute variation of dietary sodium affected the accuracy of the PAC/PRA ratio adversely; the sensitivities on and off therapy were 73% and 87%, respectively, and the specificities were 74% and 75%, respectively [27]. In a study of African American and Caucasian subjects with resistant hypertension, the PAC/PRA ratio was elevated (>20) in 45 of 58 subjects with PA and in 35 of 207 patients without PA (sensitivity, 78%; specificity, 83%) [28].
The measurement of PRA is time-consuming, shows high interlaboratory variability, and requires special preanalytic prerequisites. To overcome these disadvantages, a monoclonal antibody against active renin is being used by several reference laboratories to measure the plasma renin concentration (PRC) instead of PRA. However, few studies have compared the different methods of testing for PA, and these stud ies lack confirmatory testing. It is reasonable to consider a positive PAC/PRC test if the PAC is >15 ng/dL and the PRC is lower below the lower limit of detection for the assay.
Confirmatory Tests
An increased PAC/PRA ratio is not diagnostic by itself, and PA must be confirmed by demonstration of inappropriate aldosterone secretion [11, 12]. The list of drugs and hormones capable of affecting the RAA axis is extensive, and a “medication-contaminated” evaluation is frequently unavoidable in patients with poorly controlled hypertension despite a three-drug program. Calcium channel blockers and α1-adrenergic receptor blockers do not affect the diagnostic accuracy in most cases [11]. It is impossible to interpret data obtained from patients receiving treatment with mineralocorticoid receptor antagonists (e.g., spironolactone, eplerenone) when the PRA is not suppressed (see above). Therefore, treatment with a mineralocorticoid receptor antagonist should not be initiated until the evaluation has been completed and the final decisions about treatment have been made. The favored confirmatory test at Mayo Clinic is aldosterone suppression testing with orally administered sodium chloride and measurement of urinary aldosterone and sodium [10].
It is important to recognize that confirmatory testing is not needed in patients with hypertension and spontaneous hypokalemia when the PAC is >20 ng/dL and PRA is suppressed because there is no other disorder except PA that could be responsible for this presentation (Fig. 11.1) [11].
Oral Sodium Loading Test
After hypertension and hypokalemia have been controlled, patients should receive a high-sodium diet (supplemented with sodium chloride tablets if needed) for 3 days, with a goal sodium intake of 5000 mg (equivalent to 218 mEq of sodium or 12.8 g sodium chloride) [16]. The risk of increasing dietary sodium in patients with severe hypertension must be assessed in each case [29]. Because the high-sodium diet can increase kaliuresis and hypokalemia, vigorous replacement of potassium chloride may be needed, and the serum level of potassium should be monitored daily. On the third day of the high-sodium diet, a 24-hour urine specimen is collected for measurement of aldosterone, sodium, and creatinine. To document adequate sodium repletion, the 24-hour urinary sodium excretion should exceed 200 mEq. Urinary aldosterone excretion of more than 12 μg/24 h in this setting is consistent with autonomous aldosterone secretion [16]. The sensitivity and specificity of the oral sodium loading test are 96% and 93%, respectively [30].
Intravenous Saline Infusion Test
The intravenous saline infusion test has also been used widely for the diagnosis of PA [11, 12]. Normal subjects show suppression of PAC after volume expansion with isotonic saline; subjects with PA do not show this sup pression. The test is done after an overnight fast. It is important to be sure that the patient is normokalemic before this test because the sodium load will increase the renal excretion of potassium. Two liters of 0.9% sodium chloride solution are infused intravenously with an infusion pump over 4 h with the patient recumbent or seated (see below). Blood pressure and heart rate are monitored during the infusion. At the completion of the infusion, blood is drawn for measurement of PAC. PAC levels in normal subjects decrease to <5 ng/dL, whereas most patients with PA do not suppress to <10 ng/dL. Post-infusion PAC values between 5 and 10 ng/dL are indeterminate and may be seen in patients with IHA. Historically the saline infusion test has been performed in the supine position, and the false-negative rate has been excessive; preliminary data suggest that if the saline infusion test is performed in the seated position, the accuracy is improved [31].
Fludrocortisone Suppression Test and Captopril Stimulation Test
Subtype Evaluation Tests
After case detection and confirmatory testing, the third management issue guides the therapeutic approach by distinguishing between unilateral adrenal disease (e.g., APA and PAH) from bilateral adrenal disease (e.g., IHA and GRA). Unilateral adrenalectomy in patients with APA or PAH results in normalization of hypokalemia in all cases; hypertension is improved in all cases and is cured in 30–60% [34–36]. In IHA and GRA, unilateral or bilateral adrenalectomy seldom corrects the hypertension [16]. IHA and GRA should be treated medically. APA is found in approximately 35% of cases and bilateral IHA in approximately 60% (Table 11.1). APAs are usually small hypodense adrenal nodules (<2 cm in diameter) on computed tomography (CT) and are golden yellow in color when resected (Fig. 11.2). IHA adrenal glands may be normal on CT or may show nodular changes. In general, patients with APAs have more severe hypertension, more frequent hypokalemia, and higher levels of plasma aldosterone (>25 ng/dL) and urinary aldosterone (>30 μg/24 h) and are younger (<50 years), compared with those who have IHA [16, 37]. Aldosterone-producing adrenal carcinomas are almost always larger than 4 cm in diameter and have an inhomogeneous imaging phenotype on CT.
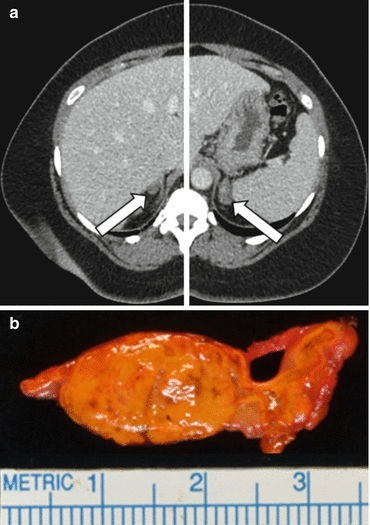

Fig. 11.2
A 49-year-old woman had a 22-year history of hypertension and 2-year history of hypokalemia. The case detection test for PA was positive, with a plasma aldosterone concentration (PAC) of 52 ng/dL and low-plasma renin activity (PRA) at <0.6 ng/mL/h. In view of the spontaneous hypokalemia, confirmatory testing was not needed. Panel (a), adrenal CT shows a 1.2 cm, low-density nodule (arrow) in the lateral limb of the right adrenal gland and a 2.2 cm low-density nodule (arrow) in the left adrenal gland. Adrenal venous sampling (Fig. 11.4) lateralized aldosterone secretion to the left adrenal gland and a yellow 2.1 cm cortical adenoma (shown in panel b) was found at laparoscopic left adrenalectomy. The postoperative plasma aldosterone concentration was <4.0 ng/dL. Hypokalemia was cured, and blood pressure was normal while taking two antihypertensive medications
Abdominal CT
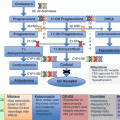
PA subtype evaluation may require one or more tests, the first of which is imaging of the adrenal glands with CT (Fig. 11.3). If a solitary unilateral hypodense (HU < 10) macroadenoma (>1 cm) and normal contralateral adrenal morphology are found on CT in a young patient (<35 years) with severe PA, unilateral adrenalectomy is a reasonable therapeutic option (Fig. 11.3) [11, 39]. However, in many cases, CT shows normal-appearing adrenals, minimal unilateral adrenal limb thickening, unilateral microadenomas (≤1 cm), or bilateral macroadenomas. In these cases, additional testing is required to determine the source of excess aldosterone secretion.
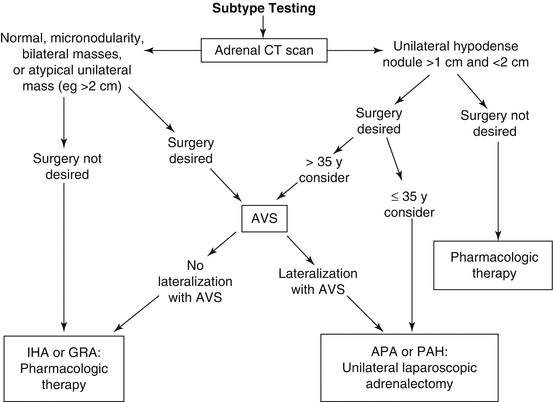

Fig. 11.3
Subtype evaluation of PA. For patients who want to pursue a surgical treatment for their hypertension, adrenal venous sampling is frequently a key diagnostic step (see text for details). APA aldosterone-producing adenoma, AVS adrenal venous sampling, CT computed tomography, IHA idiopathic hyperaldosteronism, PA primary aldosteronism, PAH primary adrenal hyperplasia (Modified from Young WF Jr., Hogan MJ: Renin-independent hypermineralocorticoidism. Trends Endocrinol Metab. 1994;5:97–106) [38]
Small APAs may be labeled incorrectly as IHA on the basis of CT findings of bilateral nodularity or normal-appearing adrenals. Also, apparent adrenal microadenomas may actually represent areas of hyperpla sia, and unilateral adrenalectomy would be inappropriate. In addition, nonfunctioning unilateral adrenal macroadenomas are not uncommon, especially in older patients (>40 years) [40]. Unilateral PAH may be visible on CT, or the PAH adrenal may appear normal on CT. Thus, adrenal CT is not accurate in distinguishing between APA and IHA [37, 39, 41]. In one study of 203 patients with PA who were evaluated with both CT and adrenal venous sampling, CT was accurate in only 53% of patients; based on the CT findings, 42 patients (22%) would have been incorrectly excluded as candidates for adrenalectomy, and 48 (25%) might have had unnecessary or inappropriate surgery [37]. In a systematic review of 38 studies involving 950 patients with PA, adrenal CT/MRI results did not agree with the findings from adrenal venous sampling in 359 patients (38%); based on CT/MRI, 19% of the 950 patients would have undergone noncurative surgery, a nd 19% would have been offered medical therapy instead of curative adrenalectomy [41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree