Diagnosis and Management of Ascites
Jay R. Thomas
Charles F. von Gunten
Epidemiology
Ascites, the accumulation of fluid in the abdomen, is common in patients with certain types of end-stage cancer. Its formation may be a direct result of a malignant process or secondary to unrelated comorbidity. Because the pathophysiology of fluid collection varies, treatment strategies differ. Clinical distinction between the causes of ascites is therefore imperative.
Of all patients with ascites, approximately 80% have cirrhosis (1). Other causes of nonmalignant ascites are accounted for as follows: heart failure, 3%; tuberculosis, 2%; nephrogenic ascites related to hemodialysis, 1%; pancreatic disease, 1%; and miscellaneous entities such as hepatic vein thrombosis (Budd-Chiari syndrome), pericardial disease, and nephrotic syndrome account for approximately 2% (1). Only 10% of patients who have ascites have malignancy as the primary cause (1). In these patients, epithelial malignancies, particularly ovarian, endometrial, breast, colon, gastric, and pancreatic carcinomas, cause over 80% of malignant ascites. The remaining 20% is due to malignancies of unknown origin (2). In one study, Runyon has shown that 53.3% of malignant ascites is associated with peritoneal carcinomatosis, 13.3% is associated with massive liver metastases, 13.3% is associated with peritoneal carcinomatosis and massive liver metastases, 13.3% is associated with hepatocellular carcinoma with portal hypertension, and 6.7% is associated with chylous ascites (3).
In general, the presence of ascites portends a poor prognosis, regardless of the cause. Patients with nonmalignant ascites related to cirrhosis have a survival rate of approximately 50% at 2 years (1). The mean survival in patients with malignant ascites is generally ≤4 months (4). However, with ascites due to a malignancy that is relatively sensitive to chemotherapy, such as newly diagnosed ovarian cancer or lymphoma, the mean survival may improve significantly (4).
Pathophysiology
Nonmalignant Ascites
The mechanisms that lead to the development of ascites are many, and controversy exists regarding which factors are most important. The most common cause of nonmalignant ascites is cirrhosis of the liver. In cirrhotic ascites, abnormal sodium retention is mediated by various hormonal and neural mechanisms, similar to those responsible for excess fluid retention in congestive heart failure. A hemodynamic state exists where total blood volume and cardiac output are increased and systemic vascular resistance is low. Studies have implicated nitric oxide as one potential mediator of this arterial vasodilation (5). In response, the vasoconstrictors of the renin–angiotensin–aldosterone system and the sympathetic nervous system are activated. Although atrial natriuretic peptide levels are increased, there is reduced renal responsiveness (6). In addition, arginine vasopressin, a potent vasoconstrictor, is activated in a manner independent of the osmotic state (7). The net result is an increase in total body sodium and water. In conjunction with cirrhosis, which has caused increased hepatic venous and lymphatic resistance, severe portal hypertension ensues. The increase in hepatic venous sinusoidal and portal pressures causes the excess fluid volume to localize to the peritoneal cavity secondary to fluid transudation from the splanchnic capillary bed. Ascites accumulation is also exacerbated by diminished intravascular oncotic pressure, resulting from hypoalbuminemia caused by decreased synthetic capacity of the cirrhotic liver.
Malignant Ascites
Malignant ascites arises through different pathophysiologic mechanisms. First, in peritoneal carcinomatosis, neovascularization and subsequent “leak” from vessels is thought to play a prime role in ascites development. Researchers have identified a vascular growth and permeability factor that increases fluid leak from peritoneal vasculature; vascular endothelial growth factor (VEGF) is a prime candidate for this activity (8). Compared with cirrhotic ascites, high levels of VEGF are present in malignant ascites from gastric, colon, and ovarian cancers (9). In animal models, inhibiting the tyrosine kinase activity of VEGF receptors reduced ascites formation (10). Matrix metalloproteinases (MMP) also appear to be involved in this process. Breaking down the extracellular matrix is an important step in neovascularization and metastatic spread. In animal models, MMP inhibitors significantly reduced malignant ascites (11). Portal pressures may be raised by direct tumor invasion of the liver with resultant hepatic venous obstruction. The resultant portal hypertension leads to transudation of fluid across the splanchnic bed into the abdominal cavity as in cirrhotic ascites. A final mechanism of ascites formation is due to lymphatic obstruction, commonly caused by lymphoma, resulting in chylous ascites.
Diagnosis
History
Patients with ascites commonly notice an increase in abdominal girth, a sensation of fullness or bloating, and early satiety. Other useful features of the history include recent weight gain or ankle swelling. Patients may describe vague, generalized abdominal discomfort or a feeling of heaviness with ambulation. They may also note indigestion, nausea, and vomiting due to delayed gastric emptying, esophageal reflux symptoms due to increased intra-abdominal pressure, or protrusion of the umbilicus.
Physical Examination
Physical examination for ascites includes inspection for bulging flanks, percussion for flank dullness, a test for shifting dullness, and a test for a fluid wave. Jugular venous distension should also be assessed, as it may indicate a potentially reversible cardiac cause of ascites.
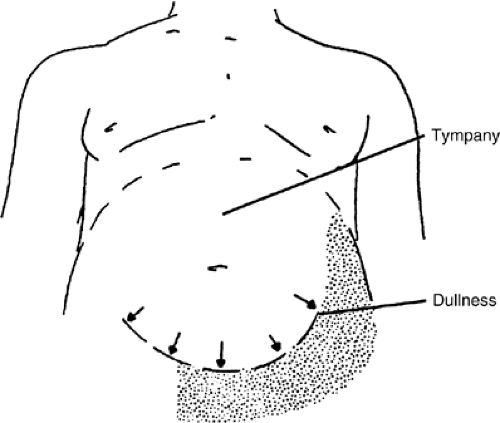
The abdominal flanks bulge when significant ascites is present because of the weight of abdominal free fluid. The examiner should look for bulging flanks when the patient is supine. The distinction between excess adipose tissue and ascites may be made by percussing the flanks to assess for dullness (Fig. 17.1)). To detect flank dullness in the supine patient, approximately 1500 mL of fluid must be present (12).
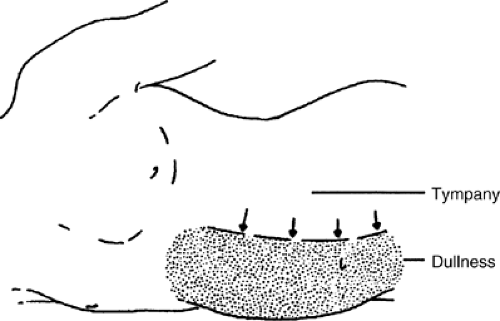
If dullness to percussion is found, examination for shifting dullness is a useful maneuver. The flank is tapped, and a mark is made on the skin at the location where the tone changes. The patient is then turned partially toward the side that has been percussed. If the location of the dullness shifts upward toward the umbilicus, it is further evidence of intra-abdominal ascites (Fig. 17.2)).
The elicitation of a fluid wave may also help to confirm the diagnosis. The test is performed by having an assistant place the medial edges of both hands firmly down the midline of the abdomen to block transmission of a wave through subcutaneous fat. The examiner places his/her hands on the flanks and then taps one flank sharply while simultaneously using the fingertips of the opposite hand to feel for an impulse transmitted through the ascites to the other flank. This test is 90% specific, but only 62% sensitive (13).
Several additional aspects of the physical examination may also be helpful. The liver may be ballotable if it is enlarged and ascites is present. If ascites is severe, the examiner may discern umbilical, abdominal, or inguinal hernias; scrotal or lower extremity edema; or abdominal wall venous engorgement. The umbilicus may be flattened or slightly protuberant. Two additional maneuvers that have been described for the physical diagnosis of ascites, the puddle sign and auscultatory percussion, are not recommended (13).
Several diagnostic tests may be useful, particularly if the physical examination is equivocal. A plain radiograph of the abdomen may demonstrate a hazy or ground-glass pattern. Ultrasonography or computed tomography scan of the abdomen can readily identify as little as 100 mL of free fluid. These latter tests are most helpful in making the diagnosis when there is a relatively small amount of fluid, or when loculation is present.
Laboratory Abnormalities
A diagnostic paracentesis of 10–20 mL of fluid is useful to confirm the presence of ascites. More importantly, it is essential to help determine its cause. Identifying the cause has profound implications on the treatment to be attempted.
To perform paracentesis, one of two locations is chosen. The first is a midline location 2 cm inferior to the umbilicus. This location is over the linea alba, which is typically avascular. The second is a location 2 cm superior and medial to the anterior iliac spine and lateral to the edge of the rectus sheath, avoiding entry into the inferior epigastric artery. Ultrasonography may be performed if the fluid is difficult to obtain, loculation is suspected, or surgical scarring is present. Previous surgery in the area of the procedure increases the possibility of the bowel being adherent to the abdominal wall.
After careful cleansing and local anesthetizing, a 2-inch, 20-gauge angiocatheter is attached to a 20-mL syringe. To minimize the risk of leaking fluid after the procedure, the Z technique is performed. The skin is displaced 2 cm relative to the deep fascia. The needle is slowly advanced while a small amount of negative pressure is intermittently applied through the syringe until ascitic fluid is obtained. The intermittent pressure helps to avoid trapping omentum or bowel against the needle tip. After the necessary amount of fluid has been obtained, the needle is withdrawn. The fascial planes overlap to prevent fluid leakage, a common complication with a more direct approach.
The color of the fluid should be noted. A white milky fluid is characteristic of chylous ascites. Bloody fluid is almost always
malignant in origin, although it may be due to abdominal tuberculosis. Initial bloody fluid that clears is more likely related to the trauma of the procedure.
malignant in origin, although it may be due to abdominal tuberculosis. Initial bloody fluid that clears is more likely related to the trauma of the procedure.
The fluid should undergo cytologic analysis, determination of cell counts with a differential, and determination of albumin and total protein concentrations. A Gram’s stain with culture can be performed if infection is suspected, but it has a low sensitivity. Inoculation of ascites directly into blood culture bottles increases the sensitivity of detecting infection up to 85% (14). The cell count, particularly the absolute neutrophil count, is useful in the presumptive diagnosis of bacterial peritonitis. If the neutrophil count is >250 cells per mL, bacterial peritonitis is presumed and empiric antibiotics should be started.
Cytology is the most specific test to demonstrate that the ascites is due to malignancy. Cytology is approximately 97% sensitive with peritoneal carcinomatosis (3), but is not helpful in the detection of other types of malignant ascites such as massive hepatic metastasis or lymphomatous obstruction of lymph vessels. Therefore, the absence of malignant cells does not exclude malignancy as a cause.
In the past, the total protein concentration has been used to classify ascites into the broad categories of exudate (total protein >25 g per L) or transudate (total protein ≤25 g per L). However, this classification system has limitations and sometimes fails to lead to optimal treatment. It has been superseded by the serum-ascites albumin gradient (SAAG). It is defined as the serum albumin concentration minus the ascitic fluid albumin concentration. The SAAG directly correlates with portal pressure (15). Patients with an SAAG of 1.1 g per dL or more have ascites that is due in part to increased portal pressures, with an accuracy of 97%. Patients with an SAAG less than 1.1 g per dL do not have portal hypertension, with an accuracy of 97% (16).
The superiority of the SAAG to the exudate/transudate characterization is shown by two examples. Cardiac ascites is associated with portal hypertension and would be expected to be transudative; however, the total protein levels in cardiac ascites are often exudative (16, 17). In this example, although total protein is not useful for primary categorization, it may be useful to identify some forms of ascites. Furthermore, ascites associated with spontaneous bacterial peritonitis (SBP) would be expected to be exudative consistent with an infection. However, SBP almost exclusively develops in low protein content ascites associated with portal hypertension, and total protein levels are typically in the transudative range (16).
Management
Overall goals for patient care should be considered before specific choices are made for managing ascites. The prognosis, expected response to management of the underlying conditions, and preferences for treatment should be established with the patient and family before any treatment plan is instituted. Each ascites treatment modality has associated burdens and benefits that deserve to be considered and discussed.
Whether ascites has a low or high SAAG is critical in determining the overall management plan. Ascites due to portal hypertension is in equilibrium with total body fluid. The most common cause of nonmalignant ascites, cirrhosis, falls within this category. Efforts to restrict salt and to affect fluid balance with diuretics are often successful. Malignant ascites may or may not be responsive to these efforts, depending on their cause. In peritoneal carcinomatosis, the SAAG is low, there is no portal hypertension, and the ascites is not in equilibrium with total body fluid (18). Consequently, salt and fluid restriction and diuretics may be of little use. Their injudicious use may result in intravascular volume depletion, diminished renal perfusion, azotemia, hypotension, and fatigue (2). However, there are high SAAG forms of malignant ascites that are responsive to salt restriction and diuretics. For example, in cases of massive hepatic metastasis, portal hypertension is present, and salt restriction and diuretics are indicated (18). One exception to this rule is nephrotic syndrome in which the SAAG is low but the ascites is diuretic responsive (19). The total protein is also low (≤25 g per L) in nephrotic syndrome and is therefore helpful in identifying this form of ascites. See Table 17.1 and Figure 17.3 for a summary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree