Background
Diabetes is a major health concern in children and youth, and has increased in incidence and prevalence in recent years . Although type 1 diabetes (T1D) accounts for greater than 80% of the cases of diabetes in youth less than 20 years old, recent large population-based studies reveal the incidence of type 2 diabetes (T2D) is rapidly increasing . The SEARCH for Diabetes in Youth study highlighted an increased prevalence of both T1D and T2D among children and youth over a 7-year period; however, the rate of increase was particularly striking for T2D. The rise in T2D incidence based on SEARCH data suggests that the number of youth with T2D may quadruple over the next several decades . Diabetic complications emerge with disease longevity, therefore the increasing diabetes rates in children and youth is alarming since many risk developing complications of diabetes in early adulthood .
Diabetic neuropathy (DN) is one of the most prevalent diabetic complications. Up to 50% of adults with diabetes will eventually develop DN, defined as a symmetric, distal to proximal loss of sensory greater than motor function . DN leads to symptoms (e.g., pain, numbness, paresthesia) that affect quality of life, and, in severe cases, contributes to fall risk, foot ulcerations, and amputation. Diabetic foot ulcers are associated with a high risk for long-term mortality. DN often develops years after a T1D diagnosis, but may be already present at the time of diagnosis of T2D. The SEARCH cohort showed that the rate of complications of diabetes, including DN, is nearly three times higher in T2D compared to T1D youth . Although T2D in adults and youths share similar pathophysiology, youth T2D has unique features characterized by rapid loss of beta-cell function and early development of diabetic complications . Longer lifetime exposure to hyperglycemia is a major risk factor for developing DN; however, additional factors (e.g., genetic markers, obesity, dyslipidemia, hypertension, inflammation) also contribute to the risk .
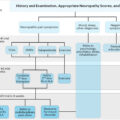
DN is infrequently reported in pediatric practice, presumably due to insensitive initial screening measures and subclinical presentations in most cases . Recent studies showed that abnormal nerve function or other DN signs can be detected in many children and youth with diabetes, even in the absence of typical DN symptoms. It is likely that DN signs are missed in routine practice, and that the number of undiagnosed cases is likely higher than currently appreciated. A careful clinical approach requires a detailed neurological evaluation and the use of sensitive techniques to detect DN in youths before reaching adulthood. More importantly, risk of progressive neuropathy can potentially be reversed by interventions such as intensive glycemic control and lifestyle changes. Beyond hyperglycemia, emerging data advocate components of the metabolic syndrome (MetS) as additional potential therapeutic targets to prevent or improve DN. Obesity is a common problem in the T2D population, and its prevalence is also on the rise in T1D children and youth . Identifying high-risk subjects would help develop preventive strategies that can halt or delay the development of DN and highly morbid conditions, such as foot ulcers and limb amputations.
Definitions and classifications
DN is described as the presence of symptoms and/or signs of neuropathy, either clinically evident or subclinical, that occurs in people with diabetes in whom other neuropathy causes are excluded. Excluding other causes of neuropathy is crucial since some are treatable or reversible . B12 deficiency should be considered in youth on a strict vegetarian diet or with pernicious anemia or metformin use. A detailed family history, including determining if family members have hammertoes or high-arched feet, is essential when hereditary peripheral nerve disorders of childhood are suspected. T1D youth are at increased risk for concomitant autoimmune neurological conditions, which can mimic DN symptoms .
An important DN characteristic in children and youth is that it may be asymptomatic or only mildly symptomatic in many patients. The current American Diabetes Association (ADA) position statement highlights the importance of early recognition and appropriate management of neuropathy in these patients . Thus a detailed neurological examination of children with diabetes is essential for discerning DN signs using sensitive techniques that detect nerve dysfunction .
In 2017 the ADA issued a position statement which classified neuropathies that occur with diabetes into four main entities: (1) distal symmetric polyneuropathy, (2) autonomic neuropathy, (3) one or more mononeuropathies (median, peroneal, ulnar, cranial nerve III), and (4) radiculopathy and polyradiculopathy (radiculoplexus neuropathy, thoracic radiculopathy) . Distal symmetric polyneuropathy is the most common type of neuropathy seen with diabetes. In this chapter, we will also refer to distal symmetric polyneuropathy as DN. DN is characterized by initial distally predominant, length-dependent impairment and loss of sensory neuron function; the later motor involvement is rarely seen in youth. If a youth presents with acute or subacute onset, proximal rather than distal involvement, asymmetrical or nonlength-dependent neuropathy, the diagnosis is not DN .
Diabetic autonomic neuropathy (DAN) is a disorder of the autonomic nervous system. DAN includes cardiac autonomic neuropathy (CAN), diabetic gastroparesis, hypohidrosis/anhidrosis, gustatory sweating, hypoglycemic unawareness, and urinary and sexual dysfunction. CAN has been studied in children and youth, and will be discussed later in this chapter.
Epidemiology
There is large uncertainty on DN prevalence and incidence in childhood and youth due to limited population-based studies in the field, wide range of clinical presentations, dissimilar methodologies used by different studies, variation in study population selection, and a lack of well-defined cohorts representative of the general target population, with the exception of a few recent studies .
The prevalence of DN in T1D youth varies widely, depending on the study population and methodology . One of the earliest studies, the Pittsburgh Epidemiology of Diabetes Complications (EDC) trial, reported DN in only 3% of T1D patients ≤18 years old, defining DN as the presence of at least two of three abnormal signs, symptoms, and decreased tendon reflexes . DN prevalence was 28% overall in the multicenter EURODIAB IDDM Complications Study . A subgroup of subjects from the EURODIAB study, aged 15–29, exhibited a DN prevalence of 19%, when diagnosing DN based on symptoms, reflex loss, and abnormalities in vibration perception threshold (VPT), and autonomic dysfunction. VPT abnormalities were reported in 62% of 339 T1D subjects aged 12–27 years in a nationwide, multicenter, cross-sectional Danish study . A summary of major studies reporting DN prevalence in T1D children and youth is presented in Table 11.1 .
| References | Study population | Methods to diagnose DN | Prevalence/incidence | Notes |
|---|---|---|---|---|
| Käär et al. Cross-sectional | Finland, clinic-based 161 T1D, >5 years | NCS (median and peroneal) | 30% peroneal and 5% median nerve abnormality | NCS considered abnormal if <−2SD |
| Maser et al. Pittsburgh EDC Cross-sectional | United States, clinic-based 400 T1D, diagnosed<17 years | Clinical history and neurological exam | <18 years: 3%18–29 years: 18%≥30 years: 58% | DN diagnosed if 2 of 3 (symptoms, signs, reflex abnormalities) present |
| Hyllienmark et al. Cross-sectional | Sweden, clinic-based 75 T1D, aged 7–20 years, duration of diabetes >3 years | NCS (median, peroneal, sural) | 57% had abnormal NCS | NCS considered abnormal if <−2 SD |
| Bognetti et al. Prospective observational | Italy, clinic-based 317 T1D | NCS (sural, median, ulnar, peroneal), performed in 292 patients | 18.5% had alterations on NCSAfter 5–7 years of diabetes in 17% of the patients | Outcomes reported based on last visit (cross-sectional); NCS abnormal if <±2 SD |
| Solders et al. Prospective longitudinal | Sweden 144 T1D, newly diagnosed, aged 4–16 years | NCS (median, peroneal, sural) | 25% abnormal sural NCV at first visit; 36.2% abnormal peroneal NCV after 10 years | Normal lower limit: mean −2.5 SD |
| Olsen et al. Cross-sectional | Denmark, multicenter 339 T1D | VPT | 62.5% abnormal VPT10–15 years: 5%15–20 years: 46.8%>20 years: 76.6% | VPT abnormal if >6.5 V |
| Nelson et al. Cross-sectional | Canada 73 T1D, duration of diabetes ≥5 years | Neurological exam, NCS (median, peroneal, sural) VPT, TPT | Abnormal exam: 36%Reduced NCV: 57%Abnormal VPT: 51%Abnormal TPT: 26% | VPT abnormal if amplitude ≥0.5 microns; TPT abnormal if the patient could not sense 1 g filament |
| Nordwall et al. Retrospective cohort | Sweden, clinic-based 80 T1D, aged 7–22 years, duration of diabetes >3 years | Clinical history and neurological exam, NCS (sural and peroneal) | 39.2% at first examination 59% after 13 years of diabetes | DN diagnosed if ≥2 of 4 NCS measurements abnormal |
| Dart et al. Clinical registry | Canada, clinic-based 1011 T1D, aged 1–18 years 342 T2D, aged 1–18 years | Health insurance records (ICD codes to detect clinically diagnosed neuropathy) | T1D: 5%T2D: 7.6% Shorter complication free survival in T2D | Neuropathy prevalence lower than most other studies likely due to methodology (the study relies on ICD coding) |
| Donaghue et al. Prospective longitudinal | Australia, clinic-based 102 T1D, aged 11–19 years, duration of diabetes >3 years | VPT, thermal threshold for heat and cold | 28.4% ≥1 abnormal test | |
| Young et al. Prospective longitudinal | United Kingdom, clinic-based 75 T1D | Neurological exam, NCS (peroneal, sural) | 32% at first examination 41% after 2.5 years | Three standardized variables summed to give a single electrophysiological variable |
| Karanavaki et al. Prospective longitudinal | United Kingdom, population-based 129 T1D | VPT, limited joint mobility | 6.2% decreased VPT 6.2% limited joint mobility | |
| Mohsin et al. Cross-sectional | Australia, clinic-based 878 T1D, aged 12–20 years, duration of diabetes >3 years | VPT, thermal threshold | 12% ≥1 abnormal test result T1: 12% T2: 19% T3: 24% | 1990–94 (T1, n =257) 1995–98 (T2, n =284) 1999–2002 (T3, n =337) Closely comparable groups |
| Eppens et al. Longitudinal | Australia, clinic-based 1433 T1D, <18 years 68 T2D, <18 years | VPT, thermal threshold | T1D: 27%, T2D: 21%, | |
| Cho et al. Observational | Australia, clinic-based 819 T1D, aged 11–17 years with 2- to 5-years diabetes duration | VPT, thermal threshold | 1990–94: 14% 1995–98: 19% 1999–2002: 28%2003–06: 23% | |
| Jaiswal et al. SEARCH Longitudinal population-based observational | Five US centers 1734 T1D, duration of diabetes ≥5 years; 258 T2D, duration of diabetes ≥5 years | MNSI exam | T1D: 7% T2D: 22%Diabetes for 5–10 years versus >10 years T1D 5% versus 13% T2D 19% versus 36% | DN diagnosed if MNSI exam score >2 |
| Amutha et al. Retrospective | India, clinic-based 3252 T1D, <20 years 899 T2D, <20 years | VPT | T1D: 8.8 /1000 person years T2D: 24/1000 person years | VPT abnormal if ≥20 V DN assessed in a small subgroup of subjects |
| TODAY2 Preliminary analysis | 517 T2D | Adjudicated neuropathy events | DN in 28%–33% of subjects by year 12 | Presented at ADA 2019 meeting |
Early reports of T2D youth with DN included few subjects. However, systematic investigation by the SEARCH study, the first US population-based study of an ethnically diverse diabetes youth cohort, reported a DN prevalence of 7% in T1D and 22% in T2D subjects with similar diabetes duration, using the Michigan Neuropathy Screening Instrument (MNSI) . Similarly, preliminary analysis of the TODAY2 study in youth revealed high rates of DN in 28%–33% of T2D subjects after 12 years . The list of major studies on DN prevalence in T2D is presented in Table 11.1 .
CAN is an independent predictor of cardiovascular mortality in people with diabetes. The SEARCH study found prevalence rates of 12% and 17% in T1D and T2D participants, respectively . In the TODAY study, the prevalence of CAN was 8% in a T2D cohort slightly younger and with lower Hemoglobin A1c (HbA1c) than SEARCH . Table 11.2 summarizes major studies reporting DAN/CAN in T1D and T2D children and youth.
| References | Study population | Methods to diagnose AN/CAN | Prevalence/incidence | Notes |
|---|---|---|---|---|
| Young et al. Prospective longitudinal | United Kingdom, clinic-based 75 T1D | HR response to DB and Valsalva; BP change in response to standing and handgrip | 19% at first examination 28% after 2.5 years | – |
| Ringel et al. Cross-sectional | United States, clinic-based 248 T1D | HR response to DB and standing | Abnormal response to either HR-DB or standing: 29%; both test abnormal: 3% | Also, follow-up data reported from 75 patients; no change in 1 year follow-up |
| Solders et al. Prospective longitudinal | Sweden 144 T1D, newly diagnosed, aged 4–16 years | HR response to DB | 25% at first visit 56% abnormal HRV after 10 years | – |
| Verrotti et al. Cross-sectional | Italy, clinic-based110 T1D | HR response to DB, Valsalva, and standing; and BP response to standing and handgrip | At least one abnormal 43% | – |
| Donaghue et al. Prospective longitudinal | Australia, clinic-based 102 T1D, aged 11–19 years, duration of diabetes >3 years | HR response to DB, Valsalva, and standing; and BP response to standing | 29.5%≥1 abnormal test | – |
| Massin et al. Cross-sectional | Belgium, clinic-based 75 T1D | HRV with 24-h Holter monitoring (five time domain and three frequency domain measures) | All indices within the normal range ≤10 years 54% ≥1 abnormal test result, ≥11 years | Two patients excluded (73 subjects analyzed) |
| Karanavaki et al. Prospective longitudinal | United Kingdom, population-based 129 T1D | HRV response to DB, Valsalva and standing; pupillometry | 15.5% 1 abnormal HRV test 7.7% ≥2 abnormal tests 7.9% abnormal pupillometry | – |
| Mohsin et al. Cross-sectional | Australia, clinic-based 878 T1D, aged 12–20 years, duration of diabetes >3 years | HRV response to DB, standing and postural BP | ≥1 abnormal test resul tT1: 18% T2: 21% T3: 18% | 1990–94 (T1, n =257)1995–98 (T2, n =284)1999–2002 (T3, n =337)Closely comparable groups |
| Eppens et al. Longitudinal study | Australia, clinic-based 1433 T1D, <18 years 68 T2D <18 years | Pupillometry | T1D: 61% T2D: 57% | – |
| Jaiswal et al. Longitudinal population-based observational | Five US centers (SEARCH) 1646 T1D 252 T2D | HRV using five domains | T1D: 12% T2D: 17% | Dysfunction defined as the presence of ≥3 of 5 abnormal HRV indices |
| Shah et al. Data from a randomized clinical trial | 397 T2D (TODAY cohort) | HRV using five domains, arterial stiffness | 8.1% versus obese controls 12.1% versus lean controls Greater pulse-wave velocity in T2D | Dysfunction defined as the presence of ≥3 of 5 abnormal HRV indices |
Clinical presentations
Diabetic neuropathy
DN is a sensory greater than motor neuropathy . Nerve damage usually begins in small fibers, defined as predominantly small diameter somatic and autonomic nerves, unmyelinated C fibers, and/or thinly myelinated A-delta sensory fibers. Small fibers carry afferent impulses that mediate pain, temperature, and unpleasant stimuli, which protect the individual from harm and also innervate the autonomic nervous system. Classically, small fiber damage causes the earliest DN symptoms, including both spontaneous and stimulus-evoked distal extremity pain. As DN progresses, large nerve fibers are affected. Large fibers are myelinated axons; these axons have different degrees of myelination and axon diameter. Large fiber damage produces tingling, loss of protective sensation, and progressive numbness.
DN symptoms of small and large fibers are frequently mild in children and youth. Commonly, the neurologic examination and nerve conduction studies (NCS), which measure only large fiber function, may uncover only subtle neuropathy signs in this age group . Other symptoms of late-stage DN include distal motor nerve involvement causing toe extension weakness, imbalance and gait problems, and altered proprioception; however, late DN symptoms are rare in children and youth. A differential diagnosis is indicated if muscle weakness develops early, for example, Charcot–Marie–Tooth disease or treatable neuropathies .
Diabetic autonomic neuropathy
DAN is a serious diabetes complication associated with an increased morbidity due to organ dysfunction, such as orthostatic hypotension, gastroparesis, hypoglycemia, and high risk of death, primarily from cardiac events. Although overt DAN is rare in diabetic children and youth, signs of autonomic dysfunction can be detected early in the disease course . DAN can affect both sympathetic and parasympathetic nervous systems; thus clinical presentation is heterogeneous and results in a variety of symptoms.
In early stages, CAN, the best studied DAN in children and youth, is usually asymptomatic, but may still be detected by standard tests, such as evaluation of heart rate variability (HRV) . As the disease progresses, CAN causes symptoms ranging from orthostatic tachycardia syndrome and lightheadedness caused by postural hypotension to silent myocardial infarctions and sudden cardiac death. Typical CAN symptoms are challenging to detect in children and youth, that may suffer from nonspecific symptoms, such as general weakness, dizziness, and fatigue. In advanced cases, CAN may be complicated by hypoglycemia awareness, a dangerous condition that poses a significant risk of severe hypoglycemia . CAN is also associated with arterial stiffness in both T1D and T2D children and youth, a well-documented risk factor that predicts future cardiovascular events .
Gastrointestinal presentations of DAN include gastroparesis, esophageal and/or gastrointestinal dysmotility, but these symptoms are rare in youth . Sudomotor dysfunction occurs as a result of sympathetic denervation and can develop distally early in the course of DN. Clinically, sudomotor dysfunction is associated with impaired sweating that can lead to dry skin, a predisposing factor to skin cracks and foot ulceration. Again, this symptom is rarely reported in youth.
Risk factors
The increasing prevalence of diabetes in children and youth necessitates urgent development of early screening strategies and improved risk factor modification to prevent DN onset and/or progression. Beyond hyperglycemia, recent studies suggest a wide range of emerging risk factors such as obesity, MetS, and genetic markers , listed in Table 11.3 along with major studies that reported them. A detailed review of DN pathogenesis can be found in two of our recent papers .