Diabetic sensorimotor polyneuropathy (DPN) and cardiovascular autonomic neuropathy, despite being very common and with heavy impact on quality of life, morbidity and mortality, remain undiagnosed in two-thirds of the cases worldwide. Thus, both effective diagnostic and preventive approaches are crucial to lower their clinical burden.
In the last decade, in addition to the setup of updated guidelines, attempts were paid at simplifying the diagnosis in clinical practice, profiling neuropathic pain, and developing sensitive biomarkers as outcome measures.
New information on risk factors and mechanisms of diabetic neuropathy supports multifactorial approach to its prevention. Lifestyle intervention and control of hyperglycemia and cardiovascular risk factors are recommended according to individual targets. Although the translation of preclinical promising results into successful therapy is still challenging and requires both novel paradigm and more research investment, pathogenetic treatment can rely on agents with preliminary positive results.
1
Introduction
Diabetic neuropathies encompass different conditions that share the involvement of peripheral nervous system but may be distinct for the nerves, fibers, and nerve roots affected, the anatomical distribution of symptoms and signs, the respective expression of sensory, motor, and autonomic abnormalities, and then the clinical appearance, the underlying pathology and pathogenesis, the temporal course, and finally the natural history and outcomes. Among the sensorimotor neuropathies, diabetic polyneuropathy is the most common (>80%) as well as one of the diabetic complications with the greatest prevalence [ ].
Definitions
The Toronto Consensus in 2009 redefined the main forms of diabetic neuropathies: (1) DPN as “a symmetrical, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvessel alterations as a result of chronic hyperglycemia exposure (diabetes) and cardiovascular risk covariates” [ ]; (2) painful diabetic polyneuropathy (PDPN) as the form of DPN with neuropathic pain arising as a direct consequence of abnormalities in the peripheral somatosensory system in people with diabetes [ ]; and (3) diabetic autonomic neuropathy as “a disorder of the autonomic nervous system in the setting of diabetes or metabolic derangements of pre-diabetes after the exclusion of other causes”, while “the impairment of autonomic control of the cardiovascular system” was the cardiovascular autonomic neuropathy (CAN) [ , ].
2
Clinical relevance of diabetic polyneuropathies
Epidemiology of DPN and PDPN
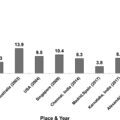
Large differences in the rate of prevalence of DPN were reported and are possibly due to sampling modalities, population characteristics, and variation in diagnostic testing and criteria. An acceptable estimate of prevalence in adults, when the clinical diagnosis was based on the presence of both neuropathic symptoms and signs, was around 30%, ranging from 13% to 54% in patients with type 1 diabetes and from 17% to 75% in those with type 2 diabetes [ ]. No major differences, at least for type 2 diabetes, were reported according to population selection, that is, hospital-based (median prevalence of 30% in type 2 and 17% in type 1 diabetes), primary care, or population screening (median prevalence of 32% in type 2 and 31% in type 1 diabetes) [ ]. However, from studies conducted in Italy, the prevalence of DPN rose from around 20% in population or primary care studies in adults older than 55 years to around 28% in specialist (secondary care) or university-based studies [ , ]. In the T1D Exchange study (5936 participants with type 1 diabetes aged 39 years and with diabetes duration of 18 years), DPN symptoms, according to the questionnaire of the Michigan Neuropathy Screening Instrument (MNSI), were present in 11% [ ].
The painful form of DPN accounts for 40%–50% of all the cases of DPN. The epidemiological findings regarding PDPN are undermined by even greater variability in diagnostic approach than for DPN, which ranges from posted questionnaires or telephone interviews to a complete clinical evaluation, and nonuniform accuracy in the exclusion of causes of pain other than PDPN. Estimates of PDPN prevalence range from 8.9% to 21.1% with a median value of about 15% from the studies where the diagnosis was based on the presence of both neuropathic pain and DPN [ ], which fulfills the International Association for the Study of Pain (IASP) criteria for probable PDPN [ ]. A cross-sectional multicenter study in Italy, including 123 participants with type 1, and 693 with type 2 diabetes (diabetes duration of 24 and 10 years, respectively) attending hospital diabetic outpatient clinics, used clinical examination and instrumental tests to get a definite/confirmed diagnosis of DPN, pure small-fiber polyneuropathy (SFN) and PDPN [ ]. It showed a prevalence of 36% for DPN, 14% for PDPN, and 2.5% for SFN [ ]. A Danish study in 389 patients with type 2 diabetes and a median diabetes duration of 5.9 years showed similar figures: definite DPN in 32.4% and definite PDPN in 13.6% [ ].
By using the validated neuropathic pain screening tool Douleur neuropathique en 4 questions (DN4), in participants from Saudi Arabia and Middle East Region (Egypt, Lebanon, Jordan, the Gulf States of Kuwait and the United Arab Emirates) a higher prevalence of PDPN than in Western populations (i.e., >50%) was documented. This was attributed to more common poor glycemic control in those areas [ ], while more recently, the prevalence of PDPN was 34.5% in Qatar [ ]. Similarly, prevalence rates of DPN in Jordan, Bahrain, Saudi Arabia, and the United Arab Emirates were between 25.6% and 58.1% with a diagnosis mainly based on MNSI [ ], while neuropathic symptoms and a vibration perception threshold (VPT) > 15 V were present in only 23% of patients with type 2 diabetes in Qatar [ ].
The rare available studies report yearly incidence of DPN from 1.7% to 3.2% in type 1 diabetes and from 2% to 8% in type 2 diabetes [ ]. A recent Danish study, in a large population at a tertiary outpatient clinic, documented a declining of incidence rate of DPN (diagnosed according to vibration perception threshold >25 V) in the period 1996 to 2018 from 4.78 to 1.15/100 person-years for a 40-year-old-men with type 1 diabetes and from 16.55 to 8.02/100 person-years for a 60-year-old-men with type 2 diabetes [ ]. This was possibly due to improved multifactorial diabetes treatment.
Epidemiology of diabetic autonomic neuropathy
Epidemiological findings mainly regard CAN prevalence assessed using the gold standard of cardiovascular autonomic reflex tests (CARTs) or other indexes of autonomic cardiovascular control as those based on heart rate variability (HRV) and baroreflex sensitivity (BRS). The prevalence rates vary largely according to the diagnostic criteria adopted, the use of age-related normative values, and the population studied [ ]. The available studies provide a prevalence of CAN around 20% in unselected patients in both type 1 and type 2 diabetes, with an increase up to 38% in type 1% and 65% in type 2 diabetes according to age and diabetes duration, respectively [ ]. The available longitudinal studies indicate an increase in prevalence of CAN of about 1.7% in type 1 [ ] and 5%–6% per year in type 2 diabetes [ ].
More recent studies have documented from one side an annual incidence of CAN lower than expected in type 2 diabetes (1.8% year) in the Denmark branch of the ADDITION study as a possible result of high-intensity treatment from the early screening–based diagnosis [ ], whereas intensive glycemic control was not able to fully prevent CAN in DCCT and EDIC studies [ ].
Moreover, despite the dependence of DPN and CAN prevalence on age and duration, they do not save young people as shown in the very large SEARCH study from US where in 1734 young participants with type 1 and 258 with type 2 diabetes, with mean age of 18 and 22 years, respectively, DPN and CAN prevalence were 7% and 12% in type 1 and 22% and 17% in type 2 diabetes [ , ]. This resembles the finding of 7.7% prevalence of CAN observed in a smaller study in participants with newly diagnosed type 1 diabetes [ ].
Ethnic differences in prevalence of DPN and CAN were described, with a saving effect in DPN incidence of being South Asians living in UK compared to Caucasians, although studies from India, Pakistan, and Sri Lanka show prevalence rates of DPN around 25% (ranging from 19% to 39% in population or secondary centers studies) and of CAN around 40%, with values ranging in small secondary centers studies from 20% to 63% [ ].
Diabetic polyneuropathies in prediabetes
Both DPN and CAN are described to start early in type 2 diabetes and to be present at diagnosis. Clinical DPN (based on impaired vibration or pressure sensation) is described in patients with newly diagnosed type 2 diabetes with prevalence rate in the largest studies between 6.6% [ ] and 16.1% [ ]. Confirmed CAN is reported in 11.7% of newly diagnosed type 2 diabetes in the KORA S4 study [ ], and 1.7% in the Verona Study where early CAN was present in 15.4% [ ].
Toronto consensus had the merit of having introduced in the definition of autonomic neuropathy the set of prediabetes [ ], and the American Diabetic Association (ADA) position statement recognized that DPN may be present in 10%–30% of subjects with prediabetes or metabolic syndrome [ ]. Despite a still open debate and not universal acknowledgment, the majority of available studies support that a clinical polyneuropathy might be present in around 10% of subjects with prediabetes (median value of prevalence of 12%, range 2%–40%, in studies with >200 participants), as small-fiber or mixed polyneuropathy [ , ]. Discrepancies in results are attributed to the variability in the diagnostic approach and in characteristics of studied populations as dimension, ethnicity, and age [ ]. A J-shaped relationship was observed between quartiles of 2-h postload glucose and the presence of clinical DPN in the KORA study [ ].
Data on the prevalence of CAN in prediabetes are not numerous and also heterogeneous regarding categories of glucose abnormalities [impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or combined IFG plus IGT], sample size, and the modalities of CAN testing [CARTs or HRV indexes, use of standardized procedures and age-related normative values, and diagnostic criteria], and very few studies include the percentage of participants with abnormalities [ ]. The majority of studies has provided limited evidence of diminished HRV indexes in prediabetes, with a trend toward worse or more widely impaired autonomic indexes in IGT versus IFG or in combined IGT plus IFG versus isolated IFG and IGT [ , ]. In the population KORA S5 study, the prevalence of CAN, according to two abnormal HRV indexes, in those with IFG, IGT, and combined IFG plus IGT, was 8.1%, 5.9%, and 11.4%, with this latter being similar to what found in newly diagnosed type 2 diabetes [ ].
Impact on the quality of life and prognosis of diabetic polyneuropathy
In addition to the adverse consequences of neuropathic pain on quality of life (mainly depression, anxiety, and sleep problems) [ , ] and of sensory and motor deficits on the risk of falls and development of foot ulcerations, DPN plays a predictive role on mortality and morbidity beyond that represented by foot ulcerations [ ]. DPN based on the nerve conduction study (NCS) or VPT or even neuropathic symptoms is described as an independent predictor for the all-cause mortality or cardiovascular events in both diabetes types after adjustment for other risk factors and confounders with a median relative risk of 1.72 and 1.65 for mortality and cardiovascular events [ ]( Table 9.1 ). A recent meta-analysis of 31 cohorts with 155,934 participants showed that a clinical diagnosis of DPN was associated with an adjusted relative risk of death for all-cause mortality of 2.36 in type 1 diabetes and 1.46 in type 2 diabetes [ ].
| Author (year; country) | Study (participants; follow-up) | Condition | Measure | Adjusted relative risk (95% CI) | Adjusted relative risk (95% CI) |
|---|---|---|---|---|---|
| All-cause mortality | Cardiovascular events | ||||
| Saluja (2020; Europe, Australasia, South-east Asia, USA) | Meta-analysis of 11 studies (446,916; 5 years) | Foot ulcer | Presence of diabetic foot ulcer | 2.45 (1.85–2.85) | Not provided |
| Hsu (2012; Taiwan) | Community-based study (326 with type 2; 5 years) | DPN | Nerve conduction study | 4.44 (2.67–8.91) | Not provided |
| Brownrigg (2014; UK) | Primary care cohort (13,043 with type 2; 2.5 years) | DPN | 10 g monofilament | Not provided | 1.33 (1.02–1.75) |
| Seferovic (2018; USA, Europe) | ALTITUDE trial (8463 with type 2 and kidney and/or cardiovascular disease; 2.6 years) | DPN | ≥1 positive answer to 3 MNSI-Q questions | 1.24 (0.99–1.56) | 1.49 (1.20, 1.85) (MACE) |
| Bjerg (2019; Denmark) | Clinical cohort study (3828 with type 1; 7 years) | DPN | Vibration perception threshold | 1.72 (1.39, 2.12) | Not provided |
| Bjerg (2021; Denmark) | ADDITION-Denmark and DD2 cohorts (1445 and 5028; 11.4 and 2.2 years, respectively) | DPN | MNSI-Q (cut-off 4) | 1.11 (0 83–1 48) | 1.65 (1.41–1.95) |
| Vagi (2021, Hungary) | Retrospective clinical cohort study (131 with type 1 and 1011 with type 2; 9 and 8 years, respectively) | DPN | Current perception threshold | 2.99 (1.4–8.63) (type 1) 1.32 (1.07–1.64) (type 2) | Not provided |
| Lapin (2020, USA) | Retrospective clinical cohort study (43,945 with type 2; 3.1 years) | Painful DPN | Electronic algorithm (DPN code plus pain medication or symptom) | 1.42 (1.25–1.61) (vs. painless DPN) | 1.55 (1.29–1.85) (vs. painless DPN) |
| Hicks (2021, USA) | NHANES population study (1195; 13 years) | DPN | 10 g monofilament | 1.72 (1.28–2.30) | 2.04 (1.25–3.32) (mortality) |
The presence of neuropathic pain seems to make the difference given that PDPN has been associated with 55% and 42% increased risk of vascular events and mortality when compared to painless DPN [ ] ( Table 9.1 ). Neuropathic pain is also linked with increased direct medical and indirect and social costs proportionally to the level of pain intensity [ , ].
Cardiovascular, gastrointestinal, genitourinary, and sudomotor symptomatic forms of diabetic autonomic neuropathy are not rare and can have consequences on QoL, glycemic control, and morbidity. Moreover, a predictive role of CAN on mortality and morbidity is well based. In a meta-analysis of 15 longitudinal studies published until 2001 [ ], the diagnosis of confirmed CAN was associated with a relative risk of mortality of 3.65, and large subsequent prospective studies showed that CAN diagnosis and CAN measures in both type 1 and type 2 diabetes, as in the EURODIAB study and the ACCORD study, were independent predictors of mortality for any and cardiovascular causes with relative risk between 1.32 and 2.95 [ , ]. CAN was also associated with perioperative instability during surgery, silent myocardial ischemia, coronary artery disease, cardiovascular morbidity, cardiovascular events recurrence and stroke (in type 2 diabetes) and proposed as a progression promoter of diabetic nephropathy, mainly in type 1 diabetes [ ]. Moreover, the size of CAN-related risk of mortality and morbidity might be influenced by the stage of CAN (early, confirmed, advanced). A recent meta-analysis of 19 studies (3679 patients with and 12,420 without CAN) for the outcome of all-cause mortality and of 16 studies (2875 patients with and 11,722 without CAN) for the outcome of cardiovascular events, reported that CAN was associated with a relative risk for all-cause mortality of 3.17 and of cardiovascular event of 3.16 with a greater risk in type 1 diabetes and with the diagnosis of confirmed CAN [ ]. Additional evidence comes from the meta-analyses and studies in the general and diabetic populations that attribute to typical CAN manifestations the role of independent predictors of all-cause mortality and cardiovascular events in diabetes with increased risk by 35% for 10 bpm increment of the in-trial heart rate in the ONTARGET study [ ], by 62% for orthostatic hypotension in the ACCORD study, by at least 60% for QT interval prolongation, and by 200% and more for the reverse dipping [ , , ].
3
Diagnosis of diabetic polyneuropathies
Despite their high prevalence and heavily adverse consequences, DPN and CAN remain the less recognized complications of diabetes.
In a large nationwide sample of 7378 predominantly primary care patients with type 2 diabetes in US, 62% of those with abnormal pressure sensation were not previously recognized by physicians as having DPN [ ]. In patients with type 2 diabetes from five rural Arkansas countries, who attended a diabetes education program, 79% of those with neuropathic symptoms had not been diagnosed with DPN [ ]. In the German population study Kora F4, in the elderly population with diabetes, 22% had reduced vibration and/or pressure sensation in the feet but 77% of those were unaware they had this complication [ ]. In the Australian community-based cohort of people with Type 2 diabetes of the Fremantle Diabetes Study Phase II, 67.9% of those who considered their feet to be normal had DPN according to MNSI, pointing to a gap between patient self-perceived foot health and the presence of DPN [ ]. In the German PROTECT study, DPN, assessed through pressure, temperature, and vibration perception and symptoms, was present in 51.1% of subjects with diabetes, and was previously undiagnosed in 69.5% of those with DPN, with 20% more undiagnosed cases with painless compared to painful form of DPN [ ]. In the cited study from Qatar, among patients with DPN 82% had not been previously diagnosed, while among those with PDPN 71.5% were previously undiagnosed [ ].
It is widely recognized that DPN, i.e., the loss of protective sensation, should be included in any foot ulcer risk stratification system and that its isolated presence identifies patients at medium risk [ ]. However, only 23% and 21% of patients with type 1 and type 2 diabetes, respectively, received foot examination in secondary care centers in Italy in 2018 [ ].
From what described so far, under-diagnosis and unawareness of DPN and CAN emerge as a very common phenomenon. Barriers to adequate guidelines implementation might be multiple and related to patient, physician, education, and health care organization. Moreover, the wide country-specific variability in diagnostic approach to DPN in Europe [ ] further documents the gap between knowledge, guidelines and practice.
Thus, in this century the trajectories that can be evidenced in the research field for DPN diagnosis are the following: (a) the attempts to simplify the screening and diagnostic approach to DPN in clinical practice while preserving the accuracy, in order to address its wide underdiagnosis; (b) the search for early biomarkers to use as outcomes in research and clinical trials; (c) the discover and proposal of new modalities that could cover the need for easy-to-use screening method and sensitive biomarkers. In addition, new research has been focused on neuropathic pain testing, on definition of its diagnostic criteria, and the identification of abnormal sensory function hallmarks to profile different pain phenotypes.
Diagnostic pathway for DPN
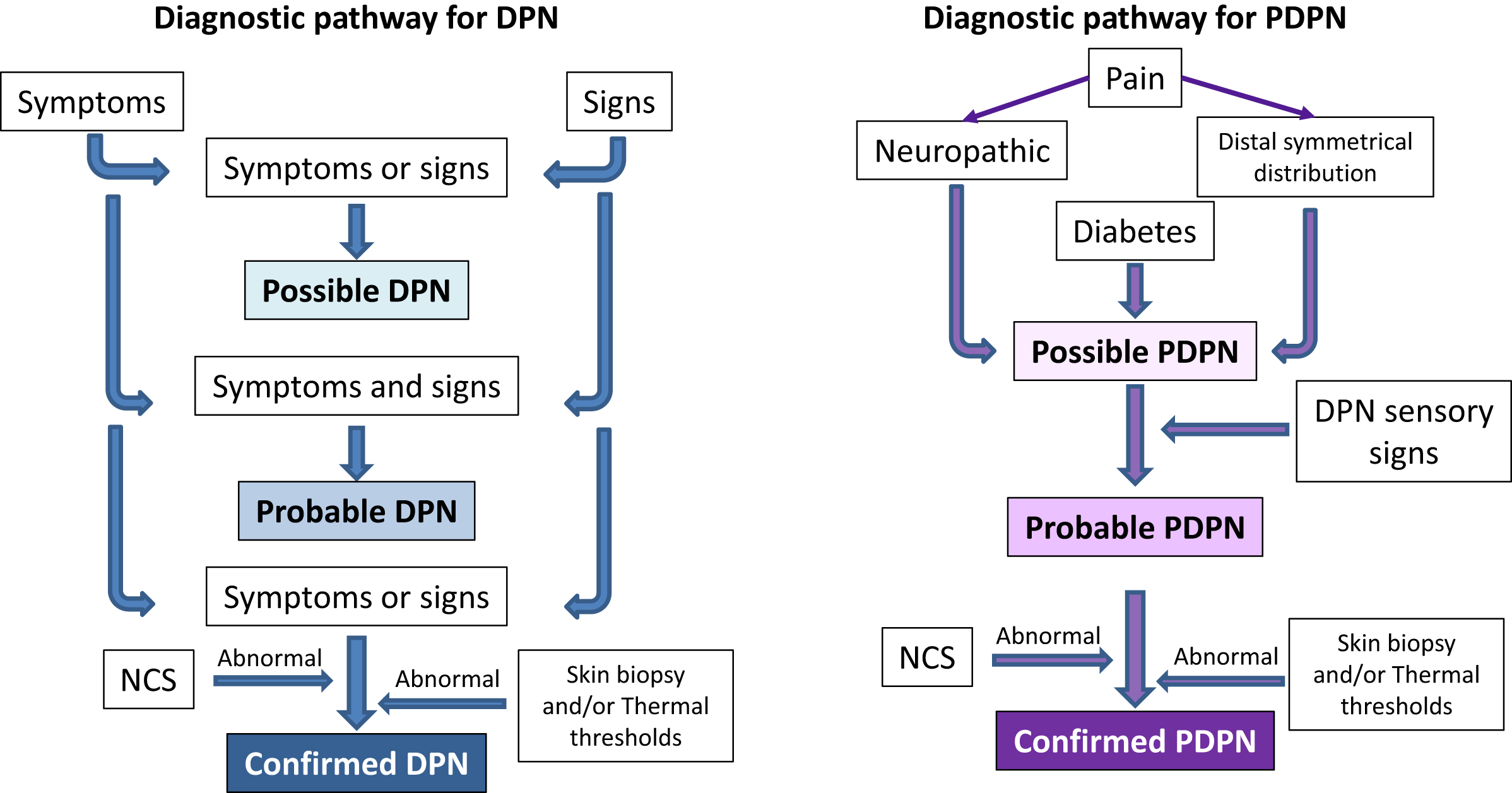
The traditional non-invasive diagnostic modalities for the peripheral neuropathies like DPN are: (1) the assessment of neuropathic symptoms, positive or negative and sensory or motor; (2) the neurological examination focused on detecting sensory deficits, motor weakness, reflexes absence; (3) the possible use of quantitative sensory testing to detect sensory deficits; and, finally, (4) the electrodiagnostic measures as gold standard [ , ]. Skin biopsy is a minimally invasive gold standard for SFN [ ]. The introduction of the concept of grading of diagnostic certainty has allowed the definition of minimal criteria to attain the diagnosis of possible, probable and confirmed DPN according to the presence of typical symptoms or signs, of both symptoms and signs, and of symptoms or signs plus NCS or small fiber function abnormalities [ , , ]. The level of probable DPN has been proposed as acceptable in clinical practice, whereas confirmed diagnosis is required in the research field. The presence of only NCS abnormalities identifies the subclinical stage of DPN ( Fig. 9.1 ).
In an effort to simplify and make more accessible the diagnosis, the guidelines developed by the diabetes societies, mostly shared by the neurological societies, have recommended easy approaches to the clinical diagnosis of DPN. They include general history and assessment of neuropathic symptoms, and with different emphasis the inspection of both feet and neurological examination (pinprick test using disposable dressmaker’s pin and/or thermal sensation for small fiber function, light touch using cotton wisp, pressure perception with 10 g monofilament and vibration sensation with 128 Hz tuning fork for large fiber function, and ankle reflex) with an indication on which patients should be evaluated (at diagnosis those with type 2, and 5 years after the diagnosis those with type 1 diabetes) and on an annual basis [ , ]. Referral to neurologist and electrodiagnostic testing should be requested in presence of atypical presentation [acute onset or rapid progression of signs, severe functional limitation, regardless of clinical pattern of affected modality, or motor predominance, or asymmetric, proximal or multifocal distribution (length independent)], or when a different cause for the clinical picture is suspected [ , ]. At least vitamin B12 deficiency and monoclonal gammopathy should be ruled out in addition to more obvious causes of polyneuropathy as alcohol abuse, uremia, and hypothyroidism.
Scoring systems for neuropathic symptoms and neurological examination
The assessment of symptoms and signs may be hampered by the low reproducibility and standardization. Composite examination scoring systems have been developed under the form of questionnaires or checklists that might favor a comprehensive, systematic and quantitative assessment by giving a guide to the appropriate questions on symptoms or to the subsequent steps of neurological examination. They are found to be reproducible and allow an overall quantification useful in the patient’s follow-up [ ].
The available systems are generally paired together for symptoms and signs and most of them have been validated in comparison with a diagnosis of DPN based on NCS and complete clinical examination. The most used are the Neuropathy Symptom Score (NSS) and Neuropathy Disability Score (NDS) [ ], the MNSI questionnaire and examination, and the Michigan Diabetic Neuropathy Score [ , ], and the Toronto Clinical Neuropathy System (TCNS) for both symptoms and signs [ ]. Some of these systems have become very popular for their reliability, ease and suitability.
Guidelines of scientific societies do not strictly prescribe the use of scoring systems for DPN assessment. However, an evidence-based report of the American neurological societies on the definition of distal symmetric polyneuropathy for clinical research stated that multiple neuropathic symptoms are more accurate than single symptoms, an examination for polyneuropathy should look for a combination of signs and that simple composite examination scores are as accurate in diagnosing polyneuropathy as complex scoring systems [ ]. Moreover, the role of questionnaires as screening tools for neuropathic pain is recognized by the IASP Neuropathic Pain Special Interest Group (NeuPSIG) [ ] and the use of assessment tools for neuropathic pain is recommended by IMMPACT in clinical trials [ ].
A recent Danish study, in 389 patients with recently diagnosed type 2 diabetes, assessed the diagnostic performance of MNSI, TCNS, Utah Early Neuropathy Scale (UENS) and DN4 plus pain in both feet toward a diagnosis of definite DPN and PDPN according to Toronto consensus criteria and NeuPSIG grading system [ ]. The study found for MNSI questionnaire score ≥4 a sensitivity of 26% and a specificity of 85%, for MNSI examination score >2 a sensitivity of 74% and a specificity of 68.5%, for TCNS score >5 a sensitivity of 63% and a specificity of 75%, for UENS a sensitivity of 83% and a specificity of 50% for the diagnosis of definite DPN, and for DN4 plus pain in both feet a sensitivity of 80% and a specificity of 90% for the diagnosis of PDPN [ ]. In comparison with the respective validation studies, these findings suggest a rather worse performance for MNSI questionnaire (compared to 40% of sensitivity and 97% of specificity [ ]) and the other tools [ , ], with the exception of DN4 interview [ ], supporting the value of this latter screening tool for PDPN.
Quantitative sensory testing
Variable intra-operator reproducibility with frequent overestimation has been reported in the neurological examination even among neurologists [ ]. Quantitative sensory testing (QST) has allowed more accurate assessment of sensory function by applying controlled and quantified stimuli and standardized procedures. Its main strength lies in the assessment of multiple sensory modalities, also those related to small fiber function not measured by electrophysiology, while QST limitations might derive from being time-consuming, expensive, in addition to its psycho dependency that makes these somatosensory psycho-physic tests a semi objective measure linked to patient’s attention and cooperation.
Although some criticism was raised in the early 2000s about the accuracy of QST for distal symmetric polyneuropathy in clinical research [ ], as well as about its diagnostic standards and utility, subsequently a NeuPSIG consensus recognized the value of QST in the clinical setting for the assessment of small and large fiber neuropathies and the monitoring of somatosensory deficits over time, in particular for DPN and with regard to thermal thresholds for diagnosis of SFN [ ]. This Consensus also recommended the use of predefined standardized stimuli and instructions, validated algorithms of testing, and reference values corrected for anatomical site, age and gender, and suggested that the clinician should apply QST with a clear understanding of the indications, methodology, limitations, and interpret the results taking into account the clinical context [ ].
Measurement of VPT by biothesiometer or neurothesiometer has been the most applied QST, while devices for the assessment of thermal perception are now more widely available. QST has gained a central role in the characterization of the somatosensory phenotypes of patients with neuropathic pain, mainly promoted by the German Research Network on Neuropathic Pain [ ].
Point-of-care NCS device
Amplitude and conduction velocity of sural nerve are among the most sensitive and valid single NCS parameter for DPN identification. NC-statDPNCheck (Neurometrix Inc., Waltham, MA), a later version of the previous NC-stat, is a point-of-care sural nerve conduction device, approved by US Food and Drug Administration (FDA), able to apply strong electric orthodromic stimuli to sural nerve and measure its amplitude and nerve conduction velocity. Its repeatability was found comparable to conventional methods, and results obtained with NP-stat or DPNCheck were found to correlate with those of standard protocol in patients with diabetes, with correlation coefficients being between 0.68 and 0.95 [ ]. A fair diagnostic accuracy was also documented for a clinical diagnosis of DPN based on NDS [ ]. A small study in 44 subjects with diabetes showed a good reproducibility and acceptable accuracy of DPNCheck for DPN based on standard NCS criteria: an amplitude of ≤6 mV had 88% sensitivity and 94% specificity for identifying age- and height-standardized reference NCS values, while a velocity of ≤48 m/s had 94% specificity and 82% sensitivity, and an abnormality in sural amplitude or conduction velocity had high sensitivity (95%) and acceptable specificity (71%). However, the study highlighted the methodological differences from standard NCS, with possible overestimation of the conduction velocity and the need to adjust the threshold values to reflect those of standard NCS [ ]. The same authors in 68 adults with ≥50 years of type 1 diabetes, confirmed the previous results (sensitivity and specificity of 86% and 79% for DPN according to standard NCS protocol, respectively), and proposed a triage model with different thresholds for amplitude and velocity that maximized sensitivity or specificity [ ].
Thus, this device might serve as an acceptable proxy to standard NCS for a more rapid and easier assessment of sural nerve function, without the need of a specialized technician for the characteristics of biosensor pads. It can also be indicated in a low resource setting in cases of diagnostic uncertainty or for research purposes [ ]. However, some technical limitations exist as the inability to detect sural amplitudes <1.5 μV that make still necessary a degree of clinical interpretation of results. Moreover, the feasibility for atypical neuropathy is unknown, and the implications of false-positive and false-negative results and the cost-effectiveness should be explored to find the best collocation of this device in clinical practice or in clinical trials for DPN diagnosis. Finally, differences in reference values according to ethnicity should be considered [ , ].
Neuropathic pain screening and assessment
Neuropathic pain of PDPN can be burning, shooting, stabbing, sharp, electric shock-like, pins and needles, splitting, lancinating, cramping, freezing, itching, toothache-like, and throbbing. It can be spontaneous or evoked, and when spontaneous continuous-permanent or paroxysmal. It is localized in a symmetrical, distal, stocking and glove pattern, first in the lower limbs, it can have nocturnal exacerbation, and can be altered by some factors such as cooling (alleviating) or plantar foot contact (exacerbating). It is associated with sensory symptoms as paresthesias, dysesthesias, hyperesthesia, allodynia (pain provoked by non-painful stimuli), hyperalgesia (exaggerated painful response to painful stimuli of slight intensity), and might affect sleep, work ability, mood, and QoL.
Pain is a subjective clinical phenomenon and patient-oriented measures can allow assessment of patient’s perspective. Self-administered questionnaires have been developed with this purpose and subjected to a complex process including validation for neuropathic pain, translation, and cross-cultural adaptation in different languages and finally a new validation of the translated versions. Pain assessment process is aimed at identifying the neuropathic origin of pain and at evaluating its qualitative and quantitative attributes. The use of validated tools for pain diagnosis, assessment, and monitoring is an essential part of the diagnostic pathway and management of PDPN [ ].
Screening tools for neuropathic pain . The screening tools for neuropathic pain are LANSS Pain Scale, DN4, Neuropathic Pain Questionnaire, pain DETECT , and ID-Pain [ ]. These tools can discriminate between nociceptive pain (expression of an adaptive protective phenomenon and evoked by high-intensity noxious stimulus) and neuropathic pain (expression of a maladaptive plasticity in the nociceptive system and spontaneous or evoked by low- and high-intensity stimuli) [ ].
The DN4, developed and validated in French and translated in many languages, is the most widely used. It consists of seven items related to symptoms (also self-report questionnaire) and three items related to clinical examination (to detect the presence of allodynia or sensory deficit in the area of pain). A sensitivity of 80%–83% and specificity of 78%–93% versus clinical diagnosis have been shown [ ] with a good performance also for PDPN [ , , ]. The painDETECT has been developed and validated in German and also available in other languages. It is a self-report questionnaire with nine items and without clinical examination, among which seven weighted sensory descriptor items (scored from never to very strongly) and two items related to spatial (radiating) and temporal characteristics of pain. A sensitivity of 85% and specificity of 80% for neuropathic pain have been shown [ ].
Diagnosis of PDPN . The diagnostic work-up for PDPN follows what suggested by NeuPSIG for the diagnosis of neuropathic pain conditions [ ]. A diagnosis of PDPN is (1) possible, in a patient with diabetes in the presence of pain with the characteristics of neuropathic pain and distribution neuroanatomically plausible with DPN (i.e., distal and symmetrical in the lower limbs), (2) probable, if pain is associated with sensory signs in the same neuroanatomically plausible distribution (i.e., sensory loss and allodynia in a symmetric distal distribution), and (3) definite, if a confirmatory diagnostic test, as NCS study and/or skin biopsy or thermal thresholds, is consistent with the presence of DPN ( Fig. 9.1 ).
The presence of neuropathic pain in the same area of symptoms and deficits of DPN meets the criteria of a probable PDPN [ , ] that is acceptable in clinical practice with the caveat of ruling out other causes of neuropathy and/or pain and of referral for instrumental investigations in case of atypical clinical presentation [ , , ].
Assessment tools for neuropathic pain . Assessment tools evaluate pain qualitative and quantitative characteristics to be monitored when evaluating the treatment response. The patient-reported outcomes (PROs) have been used for sensory profiling of neuropathic pain to define responders in clinical trials and to orient the treatment choices.
The Short-Form McGill Pain Questionnaire, although not specifically designed to assess neuropathic pain, was frequently used in the past and is now less suitable than newer tools. The Neuropathic Pain Symptom Inventory (NPSI) has been developed and validated in French, available in many languages, is a self-questionnaire that consists of 10 descriptors to discriminate, and quantify five distinct clinically relevant dimensions of neuropathic pain: spontaneous ongoing pain-superficial component (burning), spontaneous ongoing pain-deep component (squeezing, pressure), paroxysmal pain (electric shock, stabbing), evoked pain (brushing, pressure, cold stimuli), and paresthesia/dysesthesia (pins and needles, tingling), plus two items for the duration of spontaneous ongoing and paroxysmal pain. Its mean duration for filling in the questionnaire is <7 min [ ]. Brief Pain Inventory (BPI) consists of a body map, four items on pain severity providing a Pain severity index as the average of them, seven items on pain impact on function, sleep, mood, and on QoL providing a Pain interference index [ ]. Visual analog scale (VAS) or 11-point numerical rating scale (NRS) is strongly recommended to assess pain intensity and the effects of treatment both in daily practice and in clinical trials [ ].
A number of tools and questionnaires have been developed to quantify the pain impact on sleep, mood, and QoL mainly to be used in clinical trials. In clinical practice, the BPI can provide a simple measure of pain impact on QoL. Given the close relationship between neuropathic pain and depression in PDPN [ ], screening for depressive symptoms in clinical practice might be appropriate using ad hoc questionnaires as Beck Depression Inventory II or Hospital Anxiety and Depression Scale and is strongly recommended by regulatory agencies in clinical trials for pain treatment [ ].
Pain changes scales evaluate the response to treatment, such as the Patient’s Global Impression of Change (PGIC) that consists of one question about the patient’s perception of change since the start of treatment with five possible responses: very much improved, much improved, minimally improved, no change, and minimally worse.
Sensory profiling of PDPN . Mechanism/profile-based therapy claims that specific pathogenetic mechanisms of neuropathic pain may underlie different pain sensory phenotypes and then the definition of the sensory phenotype in the individual patient might allow the choice of the treatment targeted to the mechanism correspondent to his phenotype. The sensory profiling to define pain sensory phenotypes may be based on the sensory abnormalities, detected using QST, and on the pain characteristics, assessed using pain questionnaires as NPSI and is recognized as an adequate stratification tool of patients in clinical trials for neuropathic pain [ ].
QST protocol used for sensory profiling includes both thermal and mechanical test stimuli to assess gain and loss of sensory functions (i.e., the thresholds of cold and warm detection and pain, the thresholds of mechanical detection and pinprick pain, those of vibration detection and pressure pain), and different types of hyperalgesia, dynamic mechanical allodynia, and hyperpathia [ ]. This approach, including 13 parameters, allowed the definition of three main clusters of abnormalities and sensory profiles, that is, (1) the loss of all sensory fibers, (2) the thermal hyperalgesia, and (3) the mechanical hyperalgesia, which would correspond to specific pathogenetic mechanisms of neuropathic pain, that is, (1) the deafferentation, (2) the peripheral, and (3) central sensitization, respectively [ ]. A simplified sensory profiling of pain phenotypes might use bedside thermal and mechanical devices and thus could be more suitable in clinical practice and in large clinical trials [ ]. A moderate to excellent test-retest reliability has been documented for this simple bedside-QST battery [ ].
QST profiling relates to evoked sensory perceptions in experimental conditions and does not explore spontaneous pain that can be instead captured by questionnaires. Using the newly developed painPREDICT questionnaire, three different characteristic sensory symptom profiles were identified in patients with neuropathic pain (including 330 with PDPN): (1) irritable nociceptors, (2) deafferentation pain, and (3) pain attacks with nociceptive component [ ]. Moreover, NPSI allowed to identify 3 clusters of patients characterized by higher NPSI scores for pinpointed pain (cluster 1), evoked pain (cluster 2), or deep pain (cluster 3) [ ] and to find in 97 patients with neuropathic pain that the efficacy of botulinum toxin A was associated with the clusters 2 and 3 and not with the cluster 1 [ ]. This study developed and preliminarily validated a web-based version of the NPSI and an algorithm for the sensory stratification of patients in both research and daily practice [ ].
Thus, recent studies are confirming the validity of the sensory profiling approach that based on clustering of signs and symptoms is aimed at stratifying the patients’ pain phenotypes for the best treatment choices. Given the multidimensional nature of pain, in the future, these stratification systems might also incorporate genetics, psychosocial aspects, and comorbidities.
Small fiber function assessment
DPN is typically a disease of both large and small fibers. However, a pure SFN may be present in patients with diabetes with a prevalence of 2.5% [ ] as well as in nondiabetic conditions and characterized by small fiber sensory symptoms (pain, typically with burning characteristics), and signs (thermal and pain hypoesthesia, allodynia, hyperalgesia) in a symmetrical and distal distribution, reduced intraepidermal nerve fiber (IENF) density on skin biopsy, and/or reduced thermal thresholds, with normal sural NCS [ ]. The Toronto Consensus has defined the minimal criteria for possible (symptoms and/or signs), probable (both symptoms and signs plus normal sural NCS), and confirmed small fiber neuropathy in diabetes, with this latter level requiring altered IENF density on skin biopsy at the ankle and/or abnormal thermal thresholds at the foot [ , ]. In addition to QST and skin biopsy, corneal confocal microscopy (CCM) and sudomotor testing have been also applied in the diagnostic pathway of SFN in diabetes [ ].
Skin biopsy
Morphological study and quantification of IENF using skin biopsy of distal leg is accepted as a validated, safe, easy, nonpainful, economic, and minimally invasive standardized measure of small fibers, provided with high sensitivity and specificity for SFN diagnosis (based on IENF morphometric analysis), and with age and sex related normative values being available [ ]. IENF study is recognized as a valid and reliable technique for the diagnosis of peripheral polyneuropathies [ , ], and requested to confirm the diagnosis of SFN in clinical practice [ ], whereas, it can be used in research for monitoring DPN progression, evaluation of fiber regeneration, and as endpoint in clinical trials.
In diabetes, IENF density was found to be reduced also in mild forms of DPN [ ], related to pain and sensory deficits [ ], and increased after lifestyle intervention in prediabetes [ ]. Despite its sensitivity, relation with clinical DPN, and susceptibility to disease-modifying intervention at least in prediabetes, limitations are the lack of definite data on IENF abnormalities prevalence in prediabetes and diabetes and on predictivity on DPN complications [ ]. Moreover, the possibility of ethnic differences should be taken into account [ ].
Skin biopsy has been used to explore the natural history of small fiber involvement compared to large fiber in DPN and the morphological background of pain generation. There is an overall convincement that changes in function and structure of small fibers occur early in the course of DPN but no shared position on the fact that they come before the abnormalities in large fibers. The German Diabetes Study has documented an early parallel damage to both small and large nerve fibers in well-controlled recent-onset type 2 and, to a lesser extent, type 1 diabetes [ , ]. Moreover, an exclusive relationship of small fiber damage with neuropathic pain is not confirmed. Some studies had documented an association between IENF morphology and neuropathic pain of PDPN, without however a close or constant link between them or a significant correlation between pain intensity and IENF density [ ]. On the other hand, no differences in IENF density between painless and painful DPN were more recently found [ ] and instead an association between the severity of sensory deficits and painful DPN was documented [ , ]. It is possible that the distinction between painful and painless forms of DPN is not in the degree of IENF loss but instead in a lower deficit in regenerating nociceptive fibers in the painful form [ ]. Moreover, the degree of small fiber loss might constitute the background of different pain phenotypes: in patients with PDPN, IENF density was found to be higher in presence of provoked pain that might be mediated by spared and sensitized nociceptive afferents [ ]. A greater impairment in corneal innervation parameters has been reported in painful compared to painless DPN that was more evident than what observed with NCS [ ].
Thus, while probably small fiber abnormalities are always present in PDPN, from the perspective of preferential small or large fiber damage, the question as to why DPN can be either painful or painless remains unanswered [ ] and needs to be also declined in terms of pain phenotypes and sensory profiling (see above).
Corneal confocal microscopy
Cornea is richly innervated through nerve fibers from the ophthalmic branch of the trigeminal nerve. In vivo CCM is an increasingly used technique that provides images of corneal structure and nerves, including sub-basal nerve plexus, beneath the basal layer of the corneal epithelium, and by applying manual or automated image analysis morphometric measures of corneal nerve fiber (CNF) density (number/mm 2 ), length, and branch density [ ]. Advantages of CCM are noninvasiveness, rapidity (2 min), suitability for reiterative use, good reproducibility, the availability of worldwide normative reference values [ ], and the development of fully automated image analysis [ ]. CCM morphometric variables have shown moderate-high sensitivity and specificity for DPN and the same diagnostic accuracy for DPN as skin biopsy [ ], and correlation with IENF and other measures of small fiber function as cooling detection thresholds, laser doppler imaging flare technique results, and HRV [ ]. A meta-analysis of 38 studies (over 3000 participants) showed that the CNF density, corneal nerve branch density, CNF length, and inferior whorl length (a new parameter for the fiber length in the inferior whorl of the superficial nerve plexus) were significantly reduced (all P < .00001) in the patients with DPN compared to those without DPN and to healthy controls and in the patients without DPN compared with control subjects [ ]. A multicenter study, including 516 with type 1 diabetes and 482 with type 2 diabetes, showed moderate diagnostic performance for DPN of CNF length with an area under the curve (AUC) of 0.77 in type 1 diabetes and of 0.68 in type 2 diabetes, and at the optimal threshold of 12.5 mm/mm 2 values of 73% sensitivity and 69% specificity in type 1 diabetes, and at the threshold of 12.3 mm/mm 2 a sensitivity of 69% and a specificity of 63% in type 2 diabetes [ ].
CCM abnormalities have been associated with the severity of DPN [ ] and have been found more marked in painful compared to painless DPN [ ] and also associated with CAN [ ]. CCM abnormalities were present in subjects with newly diagnosed type 2 diabetes [ ], in people with prediabetes [ ], and in children with type 1 diabetes [ ]. They have been described to precede retinopathy and nephropathy development [ ] and to be able to identify patients with type 1 diabetes at risk to develop DPN [ ] and even people with prediabetes at risk to develop diabetes [ ]. Moreover, a rapid decline in CNF length has been associated with the development of foot ulceration and Charcot foot and an increased risk for the development and progression of DPN [ ]. In a neuropathy cohort for CCM, including 203 participants with type 1 and 58 with type 2 diabetes, free of confirmed DPN at baseline, CNF length was able to predict the development of confirmed DPN with the best diagnostic accuracy at year 6 (AUC 0.80) and an overall sensitivity of 67% and specificity of 71% at the baseline cut-off value of 14.1 mm/mm 2 [ ].
Thus, CCM seems to have a fair diagnostic accuracy for DPN and to be able to identify the patients at higher risk to develop full DPN with a sensitivity lower than 70%, at least with the actual definition of DPN, rather imbalanced in favor of large fiber involvement. Moreover, when compared to other neurological measures, as cold, warm, and vibration sensation thresholds, CNF length was not superior to the other measures in predicting the incident DPN (AUC of 0.77, 0.71, 0.74, and 0.66, respectively) [ ]. However, the greater feasibility of CCM makes it more suitable for routine screening than other instrumental technique as electrophysiology or QST. An acceptable CCM sensitivity for probable DPN was not found in a recent study in participants with recently diagnosed type 2 diabetes [ ].
Finally, CNF density has been described to change earlier than other neurological measures after combined pancreas/kidney transplantation (6 and 12 months) [ ], bariatric surgery in obese patients with type 2 diabetes [ ], insulin pump therapy in type 1 diabetes [ ], and omega-3 supplementation [ ].
Standardization of the technique has been significantly increased with regard to the type of confocal microscope, the method of scanning, image sampling, and quantitative analysis of images using both manual annotation (highly reliable but time-consuming) and a less sensitive automated system until the promising application of artificial intelligence-based deep learning algorithm for image classification using convolutional neural networks [ , ], with and without the use of annotated images for algorithm training. This fully automated method was shown to have at least the same diagnostic accuracy as the currently used automated image analysis system in identifying patients with and without DPN (AUC of 0.83, specificity of 87%, and sensitivity of 68%) [ ]. For his speed and accuracy, this CCM image analysis modality might be candidate for large application in clinical screening programs for DPN. An algorithm allowing a binary classification of the presence or absence of DPN has been recently developed thus increasing the screening ability of CCM [ ].
The feasibility of applying this algorithm in the clinical setting needs to be shown in further studies as well as the comparison with other screening methods and small-fiber function measures.
Simple diagnostic tools
Efforts have been made by scientific societies to simplify and render more accessible the screening approach to DPN, through the search of tests that might combine acceptable diagnostic accuracy with being not time-consuming, readily available, and suitable for different settings.
10 g monofilament . Among candidates for simple diagnostic tools, 10 g monofilament has played a central role. It has been proved to be a good predictor for foot ulceration and to identify patients at increased risk. A meta-analysis of 10 cohort studies of risk factors in the prediction of the first diabetic foot ulceration (16,385 participants, follow-up 1–4 years) showed that insensitivity to 10 g monofilament consistently predicted foot ulceration with an odds ratio of 3.18 (95% CI 2.65–3.82) [ ], and the loss of protective sensation based on 10 g monofilament is included in the stratification risk for foot ulceration [ ].
The diagnostic value of monofilament for DPN is less substantiated, depending also on the variable modalities of its use according to the number of times of application, the sites (usually, dorsum of the great toe for DPN screening and three plantar sites for ulceration risk), and the cut-off for abnormality. Data on diagnostic performance of 10 g monofilament on the dorsum of the hallux are not homogeneous with values of sensitivity of 64%, specificity of 77%, and AUC of 0.71 for NCS-based DPN at the threshold of ≤5 sensate stimuli out of 8 [ ], a very limited sensitivity (20% with a specificity of 98%) toward a clinical diagnosis of probable DPN [ ], and a sensitivity of 54% and a specificity of 74% for an NCS-based diagnosis of DPN for the insensitivity in ≥1 out of three sites in the feet (compared to 95% and 91% of NDS and 85% and 43% of 125 Hz tuning fork) [ ]. In a meta-analysis of eight studies, the pooled sensitivity and specificity of monofilament tests for detecting DPN based on NCS were 53% (95% CI 32%–74%) and 88% (95% CI 78%–94%), respectively, not supporting the monofilament test as an early sensitive test [ ]. However, in 175 patients with diabetes, a threshold of ≤5 sensate stimuli out of eight discriminated 4-year risk of confirmed DPN (based on NCS abnormalities and the presence of symptoms or signs) with AUC of 0.71 and sensitivity and specificity of 72% and 64%, respectively [ ].
Comparison between easy handheld devices . The pinprick sensation test using Neurotip (Owe Mumford, Oxford, England) on the dorsum of the great toe showed sensitivity of 44% and specificity of 89% with an AUC of 0.66 and 128 Hz tuning fork test on the same site had sensitivity of 44% and specificity of 97% with an AUC of 0.70 for NCS-based DPN, both at the cut-off of three insensate responses out of 8 [ ]. To evaluate the most suitable simple and rapid screening test for DPN, in 3883 patients with type 2 diabetes, ankle reflexes and thermal, vibration, pressure, and pinprick sensations were measured and compared with a diagnosis of probable DPN. While single tests showed a low sensitivity (from 51% of ankle reflex to 21% of pinprick) for probable DPN, the combination of tests was more sensitive and that of ankle reflexes, thermal and vibration sensation had the best balance between accuracy and time duration (2 min 44 s), thus proving suitable for underserved populations [ ].
Ipswich Touch Test . The Ipswich Touch Test is a simple, safe, quick, and easy method to detect loss of foot sensation, by lightly and briefly (1–2 s) touching the tips of the first, third and fifth toes of both feet with the index finger. The presence of ≥2 insensate areas had a sensitivity of 81% and a specificity of 96% compared to 10 g monofilament insensitivity when carried out in the clinic and of 78% and 94% when carried out at home. Thus, it can be used by nonprofessionals and might improve awareness in patients and relatives for appropriate care in case of sensation loss [ ].
Risk stratification systems for DPN . Simple screening tests have been combined with clinical and demographic characteristics of patients to construct risk models for DPN [ ], thus improving the diagnostic characteristics of 10 g monofilament, pinprick, and tuning fork over 80% for sensitivity, specificity, and AUC.
Prediction models for DPN have been proposed in type 2 diabetes to be used as stratification risk systems, being exclusively based on clinical and demographic variables. Risk equations, nomograms, and deep learning models have been developed for the prediction of DPN development in type 2 diabetes that might be useful to identify the best candidates to DPN screening [ ].
Attempts to address the barriers to DPN screening
The incorporation of foot examination into eye screening visits has been proposed in UK under the form of “one-stop” annual diabetes microvascular screening program, which included neuropathic symptoms and signs assessment using TCNS, 10 g monofilament, DPNCheck, and Sudoscan in addition to retinopathy and nephropathy screening [ ]. This formula was feasible and with high patient acceptability and uptake (91% of patients in favor of this service), and the operator was a skilled podiatrist able to refer at-risk patients to the foot protection team [ ].
There is a gap between what recommended by guidelines and what applied in clinical practice. This might recognize as possible causes a still present uncertainty and disagreement on the diagnostic approach or an insufficient attention to the feasibility of guidelines’ recommendations. A recent expert consensus considered the issue of implementation of DPN guidelines into clinical practice, pointing to multiple needs: clearer guidelines, the increase in awareness and education of patients, physicians and stakeholders, new models for DPN screening with involvement of trained members of diabetic care team and incorporation in routine procedures, and the adoption of a risk-based approach including screening for micro- and macrovascular complications [ ].
The same consensus proposed a simple algorithm for DPN diagnosis including as a first screening step the assessment of neuropathic symptoms (i.e., nonpainful symptoms and neuropathic pain, also using DN4, and pain severity) and signs (i.e., vibration sensation with tuning fork for large fiber and/or pinprick test for small fiber) and suggested as minimal criteria for DPN diagnosis in clinical practice the presence of bilateral impairment of vibration sensation with tuning fork and/or pinprick test. A more extended diagnostic workup can include assessment of thermal sensation, touch/pressure sensation, proprioception, and ankle reflex with the possible use of structured scoring systems for signs (as NDS, MNSI examination, TCNS, or UENS) and/or QST, as well as scoring systems for symptoms (as NSS and Total Symptom Score [TSS]). Pain interference with daily activities and sleep and exclusion of other causes of polyneuropathy are other components of the pathway that might end in particular cases to the confirmed diagnosis based on NCS or skin biopsy [ ].
Diagnosis of autonomic neuropathy
If the first-line assessment of autonomic neuropathy in diabetes regards different autonomic symptoms and signs, laboratory investigation includes the CARTs, based on heart rate and blood pressure reflex changes to physiologic provocations. Testing parasympathetic cardiac and sympathetic cardiovascular function plus sympathetic sudomotor function are recommended modalities to diagnose autonomic neuropathy [ ]. In diabetes, CAN assessment in clinical practice might also include ambulatory blood pressure monitoring (ABPM) and methods assessing HRV and BRS also used in research, whereas other techniques like muscle sympathetic nerve activity (MSNA) are strictly limited to research [ , ].
Autonomic symptoms
Autonomic symptoms can regard cardiovascular, gut, bladder, pupillary, erectile, and sudomotor function. Scientific guidelines [ , ] recommend symptoms assessment in any diabetic patient for their impact on QoL and in order to obtain a differential diagnosis but with some reserve on their diagnostic accuracy due to their low specificity. Moreover, the first validated structured questionnaire, the Autonomic Symptom Profile, was rather demanding with its 169 items [ ]. Easier questionnaires have been developed more recently as the Composite Autonomic Symptom Score 31 (COMPASS 31) questionnaire that includes 31 items in 6 domains of orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor dysfunction [ ]. COMPASS 31 was firstly validated toward a CARTs-based confirmed CAN diagnosis (AUC of 0.71, sensitivity of 70% and specificity of 67%) [ ]. Other subsequent studies found sensitivity between 70% and 89% and specificity between 40% and 67% for confirmed CAN at a cut-off value around 16–17 [ ] ( Table 9.2 ), thus supporting its use for a standardized and straightforward assessment of autonomic symptoms. The same diagnostic value was suggested for the Survey of Autonomic Symptoms scale [ ].
| Author (year; country) | Participants | CAN measures | CAN diagnostic criteria | COMPASS 31 cut-off | CAN | ||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | |||||
| Greco (2017; Italy) | 17 with type 1 56 with type 2 | DB, LS, VR, OH | 2 Abnormal tests | 17 | 70 | 66.7 | 0.707 |
| Singh (2019; India) | 54 with type 2 | DB, LS, VR, OH, SH | 2 Abnormal tests or OH | 16.54 | 88.9 | 40 | 0.731 |
| 28.67 | 77.8 | 71.1 | |||||
| D’Amato (2020; Italy) | 36 with type 1 66 with type 2 | DB, LS, VR, OH | 2 Abnormal tests | 16.44 | 75 | 56 | 0.732 |
| Zhang (2020; China) | 103 with type 2 | DB, LS, VR, OH | Autonomic score ≥2 | 19.5 | 67.4 | 83.3 | 0.816 |
| Peng (2021; China) | 126 with type 2 | DB, LS, VR, OH | 2 Abnormal tests | 14.72 | 63.3 | 64.9 | 0.630 |
Cardiovascular signs
Tachycardia, orthostatic hypotension, QT interval prolongation, and reverse dipping on ABPM are typical findings of CAN, which can play as an alert and can be easily detected during clinical evaluation, ECG or at an ABPM performed for other reasons. However, with the exception of tachycardia, they are specific but insensitive markers of CAN, with sensitivity being between 26% and 31% and specificity between 86% and 95% [ ]. The Toronto Consensus recommended screening for orthostatic symptoms in any diabetic patient, a yearly orthostatic hypotension test, in particular in patients over the age of 50 and hypertensive patients [as also suggested by European Society of Cardiology/European Society of Hypertension in people with diabetes [ ]], and referral to CARTs in the presence of unexplained tachycardia, QT interval prolongation, and reverse dipping [ ]. Awareness and detection of orthostatic hypotension is far from being diffused in clinical practice. Moreover, a seated-to-standing maneuver instead of the standard supine-to-upright test has been recently proposed for its better feasibility: with a suggested cut-off of 15 mm Hg for systolic and 7 mm Hg for diastolic BP drop [ ] might be used in situations where the use of the gold-standard modality is impossible.
Cardiovascular autonomic reflex tests
CARTs are the gold standard in autonomic testing as stated by the Toronto Consensus [ ] and recognized by neurological scientific societies [ ]. The Toronto Consensus recommends that diagnosis of CAN be based on the use of CARTs, that is, heart rate response to deep breathing, standing, Valsalva maneuver, and BP response to standing and that more than one heart rate test and orthostatic hypotension test are required [ ]. Moreover, the performance of CARTs should be standardized, the influence of confounding variables minimized, and age-related normal ranges of heart rate tests strictly required [ ]. CARTs allow CAN staging from possible or early CAN based on one abnormal cardiovagal test result, a definite or confirmed diagnosis of CAN based on two abnormal cardiovagal results, and severe or advanced CAN in the presence of orthostatic hypotension [ ].
Guidelines and feasibility of CAN screening and diagnosis
All the scientific guidelines in the field of CAN and other autonomic neuropathies share the indication to assess autonomic symptoms and signs as the first level in the diagnostic pathway, but they recognize a different strength to the recommendations on the use of CARTs, which are the gold standard for diagnosis for the Toronto Consensus [ ], always needed for American Association of Clinical Endocrinologists (AACE) with the American College of Endocrinology (ACE) [ ], and for American Autonomic Society with American Academy of Neurology indicated for the diagnosis of diabetic autonomic neuropathy [ ]. CARTs are not necessarily requested when autonomic symptoms and signs are present but possibly useful in asymptomatic patients for ADA [ ].
Selection of candidates . The Toronto Consensus indicates that at the very least screening for symptoms and signs should be universal, ADA suggests as candidates for the screening those with microvascular complications (and hypoglycemia unawareness). Actually, given the increasingly common underscreening of CAN and the huge numbers of potential candidates, a selection of those patients at higher risk might be useful. In this context, some scoring systems for CAN risk have been developed. In two population studies in China and Germany (including participants with diabetes), a CAN risk score was developed based on some variables (i.e., age, BMI, hypertension, heart rate, smoking, creatinine, and drugs with adverse effect on HRV) as a predictor of CAN (defined based on abnormal spectral or domain indexes of short-term HRV) with fair diagnostic accuracy (sensitivity of 75.4% and 78%, specificity of 62.5% and 81%) and a high negative predictive value (91.4% and 98%) [ , ]. Despite the limitations of these studies (the validation against a diagnosis of CAN not based on CARTs, and the studied population including mostly nondiabetic subjects), a risk stratification system for CAN might be useful for the selection of patients with type 2 diabetes at a higher CAN risk, who need to be referred to standard CARTs. In a group of 101 patients with type 1 diabetes, a CAN risk score including cholesterol, triglycerides, diastolic BP, hypertension, postprandial sweating, 10 g monofilament abnormality, retinopathy, and nephropathy showed in a second group of patients a sensitivity of 50%, specificity 73.8%, and negative predictive value 90.5% for CART-based CAN diagnosis [ ]. More recently, two distinct CAN risk scores for type 1 and type 2 diabetes were identified based on clinical variables, micro- and macroangiopathic complications and resting heart rate. At the cut-off of 4 out of 10, their sensitivity and specificity were 88% and 74% in type 1 (AUC 0.89) and 79% and 73% in type 2 diabetes (AUC 0.83), thus identifying asymptomatic people at higher risk of CAN and cutting the burden of CARTs by more than half [ ].
Simplification of testing . As for DPN, in order to get a greater accessibility of CARTs, some studies have elaborated some solutions: a simplification of CARTs battery by reducing the number of repetitions of the Valsalva maneuver and deep breathing or the number of CARTs; the use of reference ranges derived from literature (but even when obtained with a different methodology); the use of HRV indexes measured on short electrocardiography recordings (2–5 min) [ , ]; the development of handheld devices [ ] and technical advancement [like telemetry and mobile-derived approaches [ ]] [ ]. However, the choice of the best CART is not universally shared and the need for at least two heart rate tests plus orthostatic hypotension test is still the best compromise. Moreover, no conclusive data are available on the diagnostic accuracy of short-term HRV indexes compared to CARTs although they might be easier, perhaps more sensitive, and patient independent [ ]. A cross-sectional study in a large dataset of a Shanghai population estimated sensitivity and specificity of short-term HRV using the Bayesian approach, in the absence of a gold standard, and found that they were not inferior to the traditional Ewing test (both with values between 75% and 85%) [ ]. However, when analyzing the results, HRV indexes and Ewing tests do not seem to identify the same patients as having CAN, and when assuming CARTs as gold standard for CAN diagnosis the sensitivity of HRV indexes is 49% and specificity 78%. In a subgroup of DCCT/EDIC population with type 1 diabetes two time-domain indexes of HRV based on 10 s standard ECG showed a fair diagnostic accuracy toward CARTs-based diagnosis of CAN [AUC of 0.73, sensitivity of 62% and specificity of 79% for the standard deviation of normally conducted R–R intervals (SDNN) at the cut-off of 17.13 ms] [ ]. This limited diagnostic accuracy of HRV indexes might indicate that the two methods do not explore the same aspects of autonomic dysfunction, and then HRV indexes cannot be obviously considered an alternative to CARTs in clinical practice but as useful tests for risk stratification for CAN and for giving supplemental early and prognostic information to current CARTs [ ].
Moreover, on an individual level, the feasibility of HRV indexes as diagnostic tools for CAN also depends on (1) the availability of age-related normal values, given the age dependency [ , , ] and the age-related declining trajectories of HRV measures [ ], and (2) the application of standardized testing procedures as for the length of recording that is recommended to be 5 min [ , ]. Age-related and heart-rate-corrected reference values for ultra-short HRV measures [SDNN and root mean square of successive differences between normal-to-normal R–R intervals (RMSSD), obtained by 10-s ECGs] have been provided in the 1175 participants of the Multi-Ethnic Study of Atherosclerosis (MESA) study (free of cardiovascular disease and risk factors with mean age of 59 years) [ ] and in 13,943 cardiologically healthy participants, aged from 11 days to 91 years [ ]. However, the minimum time period for ultra-short HRV metrics is still under debate [ ].
Thus, any simplifications in CAN testing should meet the prerequisite of the pathophysiology relevance of the new approach, its validation versus gold standard, and the use of age-related reference values if appropriate.
Cardiovascular autonomic testing in research
In clinical research and epidemiological studies, short-term and 24 h HRV indexes and BRS are widely used and more readily available CAN tests [ ]. They can provide information about the pathophysiology and the natural history of CAN and may be used as sensitive and comprehensive endpoints in clinical trials together with MSNA [ ]. For clinical trials, evaluating a specific intervention or prognostic implications, the ADA guidelines suggest CARTs, HRV indexes, QTi, and resting heart rate [ ], although these latter have poor sensitivity and should be more indicated as prognostic markers. On the other hand, ADA considers BRS as a less accessible CAN measure [ ]. The Toronto consensus highlighted the importance of the control for confounding factors and strict standardization, with regard to respiration and blood pressure recording, and of complying with various technical requirements, while pointing to the unmet need of new noninvasive and safe CAN tests suitable for clinical research, for studying CAN pathophysiology and evaluating new therapeutic approaches [ ].
Surrogate markers for CAN
To overcome the limited accessibility of CARTs, the use of surrogate markers of CAN has been also explored and oriented naturally to small fiber function/structure as sudomotor function (see below) and CCM.
CCM parameters were found to be associated with CAN or related to its measures [ ] and in one study to have a high diagnostic accuracy for CAN with AUC between 0.89 and 0.91, high sensitivity (from 86% to 100%) but low specificity (from 56% to 78%) [ ]. However, in order to consider CCM as well as other surrogate markers as an alternative to CARTs, greater specificity, pertinence to clinical forms of CAN, and a predictive value for cardiovascular outcomes should be proven. Moreover, a possible role of CCM as an early biomarker for CAN requires larger studies, well-matched groups, and a comparison with sensitive autonomic indexes—like HRV and BRS—as well as a prospective design.
Recently, also easy measures of peripheral small fiber function as pain and thermal sensation using pinprick and TipTherm rod have been suggested as surrogate measures to exclude CAN in type 2 diabetes, showing for one abnormality among them sensitivity of 89%, specificity of 73%, and negative predictive value of 97% [ ].
Sudomotor and sweating function testing
Quantitative assessment of sudomotor function is considered a component of the diagnostic pathway of autonomic and small fiber neuropathies [ , , , ]. The testing modalities should evaluate the functional integrity of sudomotor neurons by measuring under controlled conditions the response to a standardized stimulus [ ].
Quantitative sudomotor axon reflex test (QSART)
In the most widely used QSART, the postganglionic sympathetic sudomotor fibers are stimulated through an axon reflex elicited by acetylcholine iontophoresis, and the sweat response is measured as increase in humidity through a hygrometer [ ]. QSART has the advantage of exploring four different proximal and distal sites that is more suitable for the assessment of length-dependent neuropathy. QSART has a good reproducibility also when used in patients with diabetic neuropathy [ ]. However, when using the commercially available device Q-SWEAT (WR Medical Electronic, Minneapolis, US), in patient with IGT neuropathy, the reproducibility was found mitigated [ ]. Disadvantages are the possible confounding effect of drugs (mainly, anticholinergic agents), the costs, and the technical complexity that limits its use to specialized laboratories [ , ]. Moreover, while a sensitivity between 64% and 80% for SFN was attributed to any abnormality in QSART [ ], in a recent study in 47 patients with type 2 diabetes and with and without diabetic neuropathy and 16 control subjects, QSART diagnostic accuracy was insufficient to differentiate between the three groups [ ].
Thermoregulatory sweat test (TST)
TST evaluates both preganglionic and postganglionic integrity (in particular, the anterior preoptic nucleus of hypothalamus, and the intermediolateral column, pre- and postganglionic efferent C-fibers) and is indicated when a central preganglionic alteration of sudomotor function is suspected [ ]. TST measures the topographic distribution of sweating indicated by the change in color of an indicator powder such as iodine with starch, quinizarin, or alizarin-red in response to a rise in core body temperature in a humidity-controlled preheated room (45–50°C) [ ]. Combination of TST and QSART is considered the gold standard for sudomotor function assessment, but TST has even higher technical demand than QSART, it is time-consuming and possible cause of patient’s discomfort [ ].
Sympathetic skin response (SSR)
SSR measures changes in electrodermal activity, which originates from sweat glands and adjacent tissues, evoked by a stimulus as an inspiratory gasp or usually an electrical stimulation through a polysynaptic reflex with a spinal, a bulbar and a suprabulbar component [ ]. Despite its simplicity, so that it can be performed in electrophysiology laboratories, and its wide use, also for diabetic neuropathy [ ], many factors hamper its clinical utility as an unclear definition of normality, high variability, tendency to habituation, dependency on age, and other confounders [ ]. Moreover, it should be considered only a surrogate measure of sympathetic cholinergic sudomotor function [ ].
Silicone imprint
This method shares similar mechanisms as QSART with lower costs and complexity. It measures manually or using a software the imprints in a thin layer of silicone caused by sweat droplets after iontophoresis of acetylcholine and is subjected to silicone related artifacts [ , ].
Quantitative direct and indirect test of sudomotor function (QDIRT)
QDIRT measures the axon-reflex sweating following iontophoresis of acetylcholine with the difference, compared to QSART, of using the color change of an indicator dye, induced by the sweat droplets, captured by digital photographs every 15 s for 7 min, and analyzed by a software to assess the response over time [ ]. Thus, QDIRT assesses both the direct (the sweating in the area in contact with acetylcholine) and indirect sudomotor response (the axon-reflex mediated sweating in the area surrounding that in contact with acetylcholine) with spatial resolution similar to silicone impressions and with temporal resolution similar to QSART [ ]. It was validated against silicone imprint and QSART with a better correlation with the silicone imprint [ ]. QDIRT has the potentiality of a greater accessibility than QSART but still requires trained personnel and strict control of room temperature and humidity, technical simplification for use outside autonomic laboratories, and more studies to define normative values and comparison with established techniques [ ].
Sensitive sweat test (SST)
SST measures postganglionic function providing temporal and spatial assessment of secretions of individual sweat glands in response to iontophoresis of pilocarpine. It uses a miniature video camera that records each iodine-starch-spot and a software that calculates secretion rate of individual sweat glands, total sweat volume, and number of secreting glands at four body sites. The pilocarpine does not activate an axon reflex and directly stimulates M3 receptors of sweat glands. This technique seems less complex than QSART, but the preliminary data, mainly available in healthy subjects, require further validation [ ].
Spoon test
This is a very easy nonquantitative screening test that assesses the smoothness sliding of the convex side of spoon on moist skin. It was compared to TST with acceptable values of sensitivity and specificity at forehead, neck, and chest sites [ ]. Being inexpensive and easy to perform, it is suitable for clinical bedside assessment of sudomotor activity but only at the chest, neck, and forehead.
Electrochemical skin conductance
The gold standard QSART and TST are complex and highly demanding tests from a technical and setting perspective and reserved to specialized laboratories [ ]. ESC measurement using Sudoscan (Impeto Medical, Paris, France) has been proposed as a reliable and easy-to-use modality for sweating function assessment mainly in diabetic neuropathy. ESC quantitatively detects the changes of electrochemical carriers on the skin surface, with a technique based on reverse iontophoresis and chronoamperometry measuring the chloride ion concentration. Applied electric current (incremental on the anode) to the skin of the hands and palms induces a shift of Cl − from the sweat glands to the skin surface, resulting in a current between the anode and a reference electrode, which is proportional to the skin Cl − concentration [ ]. Two mechanisms are proposed for this response: activation of the sympathetic sudomotor fibers (cholinergic and adrenergic) with consequent release of Cl − , or direct stimulation of sweat gland. These support the physiological justification of ESC as a measure of sudomotor function or just of sweat glands function [ , , , ]. The question of whether reduced ESC is the consequence of sudomotor fiber loss, reduced numbers or volume of sweat glands, or sweat gland dysfunction seems to be still incompletely resolved without necessarily diminishing the test validity [ ]. In 14 patients with type 2 diabetes, feet ESC showed good repeatability and reproducibility with a mean coefficient of variation of 6.9% for both [ ]. Normative values are available and document the absence of gender or BMI dependency but a slight decrease with age and an ethnicity influence [ ].
ESC for the diagnosis of SFN . Two recent retrospective studies have also evaluated the diagnostic performance of ESC for SFN, with no homogeneous definition. One study found in 210 patients referred for SFN evaluation that weight adjusted feet ESC had an AUC of 0.73 with 64% sensitivity and 77% specificity to predict abnormal sweat gland nerve fiber density (SGNFD) measured by skin biopsy and an AUC of 0.63 with 69% sensitivity and 55% specificity to predict abnormal epidermal nerve fiber density (ENFD) [ ]. Another study found in 245 patients tested for symptoms compatible with SFN, that feet ESC had a sensitivity of 60% and specificity of 89% for SFN diagnosis based on six tests [skin biopsy, QST, laser evoked potentials (LEP), CARTs, ESC, QSART] [ ]. This study also proposed the combination of skin biopsy, LEP, thermal QST, and ESC as that having the best diagnostic performance with a sensitivity of 90% and a specificity of 87% [ ]. When comparing these six methods, QST had the best sensitivity and CARTs the best specificity for SFN diagnosis. However, the tests whose diagnostic performance was under evaluation were the same used for the diagnosis [ ]. A previous study in 87 patients with and without clinically defined SFN had found that the combination of LEP, warm detection thresholds, and ESC was an effective approach to the diagnosis of SFN, but in that study, skin biopsy was not used to confirm SFN [ ]. This study showed a worse diagnostic performance of SSR compared to ESC (AUC of 0.58 vs. 0.72, sensitivity of 33.3% vs. 49.4%, and specificity of 77.6% vs. 92.5%, respectively) [ ].
ESC performance in functional-pathological studies . In functional-pathological studies, using skin biopsy parameters or CCM measures, mainly in patients with SFN, ESC was reported to be related to morphology of small fibers in a few studies although less strongly than expected [ , , ] and not unanimously [ ]. In more details, a prospective blinded study in 82 patients with SFN evaluated the relationship between ESC at the feet and skin biopsies including ENFD and SGNFD at the distal leg and found that feet ESC adjusted for weight was reduced in subjects with abnormal ENFD and SGNFD and that there was a correlation of feet ESC with ENFD and SGNFD ( P = .0001 for both) [ ]. Moreover, the areas under the curve of feet ESC adjusted for weight were 0.74 for ENFD and 0.77 for SGNFD, whereas AUCs of QSART were much lower. It is worth of note that only feet ESC adjusted for weight reached significant association with morphological measures, this was attributed to statistical reasons, differences in the plantar surface area related to weight, and the dependence of ENFD and SGNFD on weight [ ]. This latter study also highlighted a greater AUC of weight adjusted feet ESC for SGNFD than for ENFD attributed to the fact that ESC and SGFND assess similar types of fibers, although their correlation is limited by the different sites—sole and calf—of assessment and sampling [ ]. However, when considering the degree of correlation between ESC and SGNFD or ENFD to derive pathophysiological insights into the meaning of ESC, attention must be paid to some technical aspects as the limited selectivity of pan-neuronal markers used for quantitative analysis of somatic and all autonomic small fibers (also those supplying vessels and pilomotor muscle) and the possible absence of sweat glands in the skin biopsies [ , ]. Moreover, although present in few studies [ , , ] a correlation between IENFD and SGNFD is not very close, suggesting that a simultaneous involvement of somatic and autonomic nerve fibers is not the rule at least in nondiabetic neuropathies [ ]. In another retrospective one-center study, in 63 patients with a suspected involvement of small nerve fibers, among which 71% resulted to have also an involvement of large fibers and 29% a pure SFN, 41% a known cause of neuropathy and 59% an idiopathic sensory neuropathy, ESC was related to ENFD and SGNFD but only after adjustment for weight [ ], while neither EFND and SGNFD was related to weight in contrast with Porubcin et al. [ ] and adjustment of correlations between ESC and IEFND and SGNFD for body surface area weakened the correlations [ ].
It is also of note that in patients with SFN, the correlation between QSART and IENFD cannot be taken for granted [ ], and this was attributed to different sites explored by the two techniques (foot and ankle), their different functional/anatomical pertinence, and the higher susceptibility of QSART to confounding factors.
ESC for the diagnosis of diabetic neuropathy . A number of studies aimed at validating ESC measurement for the diagnosis of both DPN and CAN, with values of sensitivity from 20% to 97% and specificity from 55% to 96% for DPN [ , , ]. Data on diagnostic performance of ESC for CAN are limited with values from 60% to 91% for sensitivity and 30%–76% for specificity [ , , ] and characterized by various diagnostic modalities for the number of CARTs used [from 2 [ ] to 4], the criteria for abnormality, an inconsistent use of age-related normative values for CARTs [ ], and the substitution of feet or hands ESC measures for a scoring measure, called CAN risk score, based on both ESC results and clinical parameters [ , ] ( Table 9.3 ).
| Author (year; country) | Participants | CAN measures | CAN diagnostic criteria | CAN | ||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | ||||
| Yajnik (2013; India) | 232 with type 2 | DB, LS | 2 Abnormal tests | 92 a | 49 a | 0.740 |
| Selvarajah (2015; UK) | 45 with type 1 | HR, DB, LS, VR, OH | 1 Abnormal test | 60 | 76 | 0.660 |
| He (2017; China) | 75 with type 2 | DB, LS, VR, OH | Autonomic score ≥4 | 80 | 60 | 0.747 |
| Yuan (2018; China) | 9 with type 1 | DB, LS, VR | 2 Abnormal tests | 90.5 b | 29.5 b | 0.690 |
| 94 with type 2 | ||||||
| D’Amato (2020; Italy) | 36 with type 1 | DB, LS, VR, OH | 2 Abnormal tests | 83 | 57 | 0.916 |
| 66 with type 2 | ||||||
| Wegeberg (2020; Denmark) | 56 with type 1 | DB, LS, VR | 2 Abnormal tests | n.p. | n.p. | 0.720 |
| Lai (2021; China) | 90 with type 2 | DB, LS, VR, OH | 2 Abnormal tests | 82.6 | 40.6 | 0.674 |
a Considering CAN-risk score (based on both ESC results and clinical parameters) at the cut-off value of 35%.
ESC as outcome in clinical trials in diabetes . ESC has been found a sensitive outcome in a few intervention studies with bariatric surgery in participants with prediabetes and type 2 diabetes [ ] or with vitamin B12 [ ]. However, no association was found between ESC and CARTs at 1-year follow-up in 37 subjects with type 1 diabetes and free of complications, and the ESC decline was unrelated to clinical variables and standard autonomic measures [ ].
In summary, when considering ESC there is the paradox of a test that seems supported by the evidence of a favorable diagnostic profile as a marker of distal autonomic function, but whose endorsement as a true test of autonomic function is still questioned [ , ] with an open debate as to whether the sweating response to current in the soles and palm is expression of the number of preserved sweat glands or the efficiency of autonomic circuits and fibers, and on how ESC is affected by confounding factors.
Moreover, the ADA did not recommend a routine screening for sudomotor dysfunction in clinical practice [ ], while neurological societies include sudomotor function assessment, with QSART and TST, in the screening battery of autonomic testing [ ].
Indicator plaster
An indicator plaster (Neuropad, TRIGOcare International) works as a sweating detector through color change, it is very easy to use and the test can be also done at home: with patients lying down for 10 min with his feet undressed, two adhesive indicator tests are applied on the plantar surface of the first or second metatarsal head of both feet, the response at 10 min is normal if complete change of color from blue to pink in both feet occurs, abnormal if the response is absent or incomplete in at least one foot, the time in sec until the complete color change can be recorded (for max 30 min). This test has been proposed as a screening tool for DPN and a meta-analysis including 3470 patients showed an average sensitivity of 86% (range 42–100) and a specificity of 65% (range 22–100) for a clinical diagnosis of DPN [ ]. In 2018 and 2022, a NICE’s medical technologies guidance, while confirming its high sensitivity (89%) and limited specificity (60%) for DPN, concluded that the case for adopting Neuropad to detect preclinical DPN is not supported by the evidence, because the clinical importance of identifying sub-normal sweating and preclinical DPN is poorly defined and the cost modeling is uncertain and suggests further research addressing the comparative predictive value for foot problems of various point-of-care strategies including Neuropad, the benefits of Neuropad in vulnerable people with cognitive dysfunction or limited access to foot clinics in whom 10 g monofilament is not possible, and in general the benefits of detecting preclinical DPN [ ]. A recent longitudinal study documented in 308 patients with diabetes followed for 6 years that an abnormal indicator plaster response had independent predictivity for the first ulcer development with a high sensitivity (86%) and low specificity (49%), whereas NDS ≥6 and VPT ≥25 V had low sensitivity (40% and 39%) and high specificity (87% and 89%) [ ]. On the other hand, when considering CAN, sensitivity of the indicator plaster was found between 59% and 89% and specificity between 27% and 78% toward a CARTs based diagnosis of CAN and using a categoric modality yes/not at the 10-min observation [ ], with improvement in sensitivity by prolongation of the length of observation (15 min) [ ], or using the response as a continuous variable as the time to complete color change or as a continuous output to get the percentage color change in pink over the whole area of plaster, obtained visually [ ] or with a scanner and an image analysis software (Sudometrics) [ ]. In 51 patients with diabetes, time to complete color change was related to both autonomic and somatosensory measures, more strongly to Valsalva ratio, MNSI-Q, orthostatic hypotension, and cold thermal threshold (all P < .0001) and showed moderate diagnostic accuracy for both CAN and DPN wit AUC of 0.71 and 0.76, respectively. When prolonging the time of observation from 10 to 15 min, the specificity improved from 27% to 52% and from 31% to 62% for CAN and DPN, respectively, preserving a high sensitivity (from 82% to 82% and from 85% to 80%) [ ]. An association between IENF density and a graded indicator plaster response was also reported [ ] as well as a high diagnostic accuracy of indicator plaster for CNF length (<14 mm/mm 2 ) with AUC of 0.85, sensitivity of 83% and specificity of 80% [ ].
Cost effectiveness of CAN assessment
A common consideration about CAN is that there is no reason to look for it in the absence of symptoms and no evidence for cost-effectiveness of testing. Actually, the Toronto Consensus while recognizing that there is a lack of prospective studies assessing the cost-effectiveness of CAN testing, recommended CAN assessment in asymptomatic patients for risk stratification for diabetes-related complications, cardiovascular morbidity, and mortality, to address clinical inertia and foster patient adherence and for defining and tailoring of targets of diabetes therapy [ ]. A Korean study showed in participants with type 2 diabetes followed for 9.5 years, that the percentage of episodes of severe hypoglycemia increased from 5.4% to 17.2% and 22.7% in patients without CAN, with early and definite CAN [ ]. Moreover, two studies from Denmark and Korea showed a certain grade of reversibility for CAN in type 2 diabetes even at the stage of confirmed CAN [ , ], supporting the value of early identification and multifactorial preventive approach.
Conclusion on CAN assessment
CAN assessment is based on the evaluation of symptoms and signs with CARTs still being the gold standard for the diagnosis. The point of CAN diagnosis accessibility is crucial given the wide underdiagnosis. A reasonable diagnostic approach to CAN might be based on selection of candidates, simplification of testing, a stepwise approach to screening/diagnosis, starting on actual resources, with the caution of safeguarding the pathophysiological and clinical pertinence, as well as the accuracy of the chosen pathway ( Fig. 9.2 ).
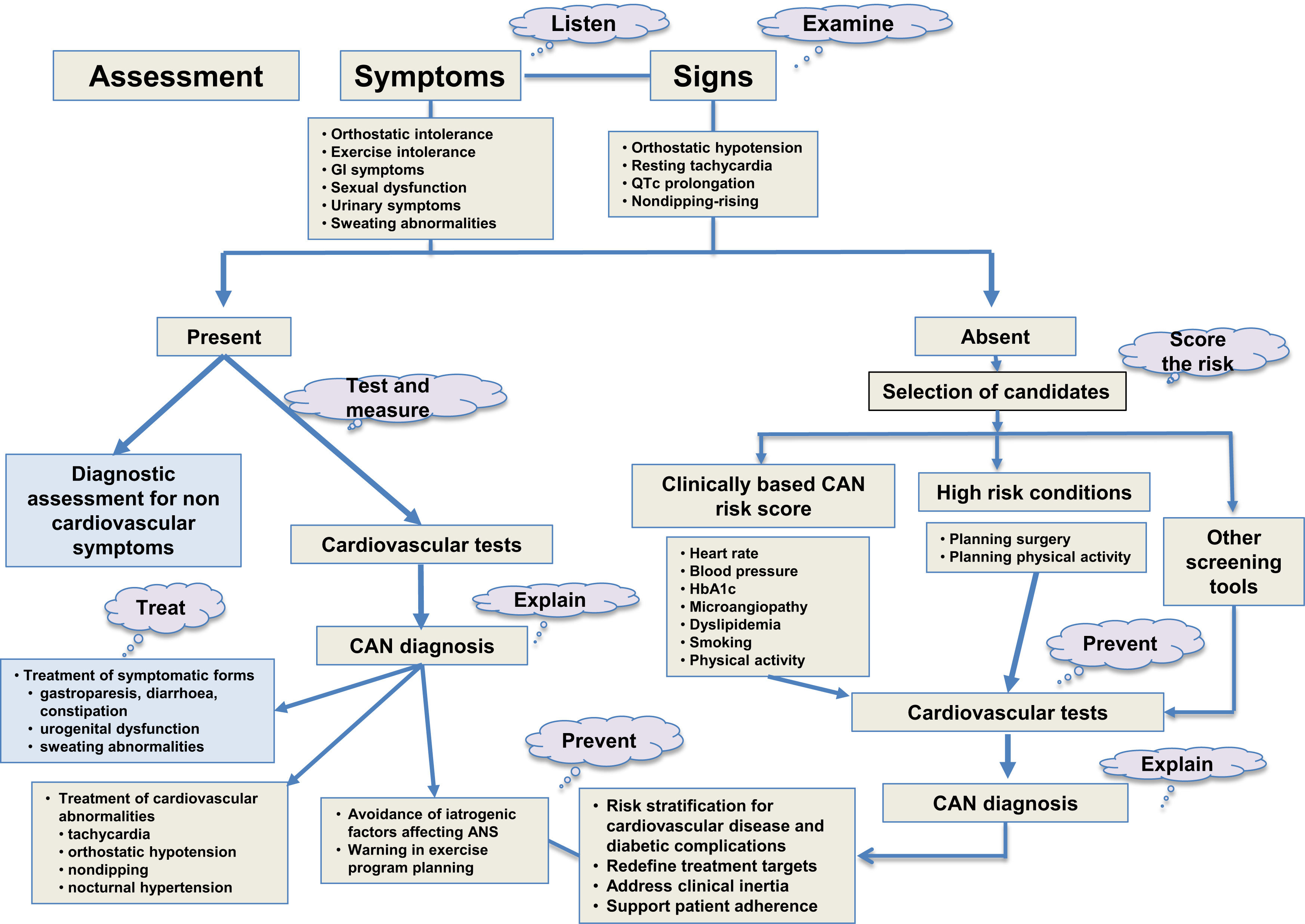
The promotion of increased awareness of the usefulness of CAN assessment in clinical practice, and the identification of barriers to implementation (burden, costs, inertia, feeling of uselessness, and unaddressed educational needs) might help in contrasting the idea that CAN assessment is only for specialized laboratories and the risk of oblivion. Resting tachycardia, QTc prolongation, and orthostatic hypotension can be a feasible approach in less served situations and at primary care level [ ].
4
Prevention of diabetic neuropathy
The pathogenesis of diabetic neuropathy recognizes multiple factors and mechanisms, which include abnormalities in lipid metabolism in addition to hyperglycemia, and the downstream effects of oxidative stress, endothelial dysfunction, endoplasmic reticulum stress, mitochondrial dysfunction, inflammation, impaired nerve function and gene expression, and DNA damage [ ]. Dyslipidemia is increasingly recognized as a pathogenetic factor of nerve damage due to its effects on advanced glycation end-products (AGE), proinflammatory action of oxidized LDL, and to the excess of nonesterified fatty acids that induce bioenergetic failure, increased reactive oxygen species production, and mitochondrial damage [ ].
Risk factors for diabetic neuropathy
The multifactorial pathogenesis of diabetic neuropathy is supported by the numerous risk markers and factors associated with its development. In addition to diabetes duration and glycemic control, hypertension, blood pressure and smoking (these last three mainly in type 1 diabetes) are the best documented, while obesity, BMI, waist circumference, and dyslipidemia (high cholesterol, low HDL cholesterol, and high triglycerides) are new emerging factors. Less strong clinical predictors of DPN are low insulin levels and insulin treatment in type 2 diabetes, low C peptide levels in type 1 diabetes, and vitamin D deficit. In patients with newly diagnosed type 1 or type 2 diabetes in the German Diabetes Study, the phenotype characterized by severe insulin-deficient diabetes was associated at baseline and at 5-year follow-up with the highest prevalence of confirmed DPN [ ]. Finally, three meta-analyses of 6, 10, and 13 cross-sectional studies, respectively, in patients with type 2 diabetes have documented that vitamin D levels were lower in DPN patients compared to those without and vitamin D deficiency was associated with an increased risk of DPN in type 2 diabetes with odds ratio of 1.07, 1.22, and 2.88 [ ]. After adjusting for age, BMI, activity score, and sunlight exposure, vitamin D levels were lower in patients with painful compared to those with painless DPN and were the most powerful predictor of the presence of PDPN [ ]. A large Iranian study reported a nonlinear relationship between vitamin D levels and DPN with an increased risk of having symptomatic DPN with both values of 25OH-D <20 ng/mL and >40 ng/mL [ ], similarly to what observed in a Danish study with regard to CAN [ ]. Recent longitudinal population or large cohorts studies as the ADDITION study have confirmed the predictive role of BMI for the incident possible DPN, while in a subgroup of the same study, HbA1c at baseline and its rate of increase predicted the incident confirmed DPN at 13-year follow-up with a 66% increased risk for 1% of HbA1c [ , ].
Little data are available for PDPN, and in studies where painless and painful DPN were concomitantly explored, diabetes duration and age were found to be correlates or predictors of PDPN compared to painless DPN in a minority of studies. A relation with the current glycemic control was documented in very few studies. The female gender was associated with PDPN but not in all studies. Obesity, weight/BMI, and abdominal obesity were found to be related to PDPN and not to DPN in a few studies. Isolated associations of PDPN with hypertension, physical inactivity, or metabolic dyslipidemia were described [ , , , , , , ]. Recently, the EURODIAB Prospective Complications Study showed in type 1 diabetes that female sex was a risk factor for incident PDPN compared to painless DPN with an odds ratio adjusted for duration and HbA1c of 2.69 while microalbuminuria was a protective factor [ ].
The risk markers or factors of CAN are almost similar to those of DPN: age, diabetes duration, glycemic control, blood pressure or hypertension, smoking, LDL, HDL, triglycerides, weight, BMI, or obesity (in type 2 diabetes), waist circumference, high insulin levels (in type 2 diabetes), cardiovascular disease, and use of antihypertensive drugs. Longitudinal studies in type 2 diabetes have confirmed the independent predictive role of age, HbA1c, BMI, and triglycerides. More recently, cross-sectional studies have documented the association of CAN with low vitamin B12 levels in type 2 diabetes [ ], and with both low and high vitamin D levels in both types of diabetes [ , ].
Biomarkers of putative pathogenetic mechanisms of diabetic neuropathy as oxidative stress, inflammation, and endothelial activation have emerged very recently as novel predictors of incident diabetic neuropathy or its progression and of deterioration of neurological measures of both DPN and CAN in type 2 diabetes such as superoxide anion generation [ ], tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1 receptor agonist, adiponectin [ ], and soluble intercellular adhesion molecule-1 (sICAM-1) [ ]. A preferential association between inflammatory biomarkers and PDPN compared to painless DPN has been observed for C reactive protein, sICAM-1, and TNF-α [ ] and a recent meta-analysis confirmed a pooled significant difference for TNF-α [ ].
Recently, glucose variability has been implicated in both DPN and CAN, without homogeneous results in particular in type 1 diabetes, while in type 2 diabetes an association of both short-term (coefficient of variation in continuous glucose monitoring) and long–term glucose variability (based on HbA1c change) with an increased risk for diabetic neuropathy seems more consistent [ , ]. Increased oxidative stress has been advocated as a possible mechanism driven by acute glycemic excursions with consequent AGE formation, the activation of the nuclear factor κ-light-chain-enhancer of activated B cells and protein kinase C (PKC) pathways, and vascular endothelial damage [ ]. The questions whether glucose variability acts independently on HbA1c, is a consequence of or a risk factor for diabetic neuropathy or a marker of disease severity remain unanswered.
Natural history of diabetic neuropathy
In the ADDITION-Denmark cohort, different trajectories in the CAN course were identified in 299 subjects with screen-detected type 2 diabetes with a consistent number of participants progressing to CAN but also 8 out of 29 subjects with confirmed CAN (at 6 years from diabetes diagnosis), who changed their status to the absence of CAN at 13-year follow-up [ ]. A retrospective longitudinal study including 759 subjects with type 2 diabetes and CAN, free of cardiovascular disease, followed for 2–3 years, showed that 226 (29.9%) had a partial recovery (i.e., a one-step improvement in CAN stage) and 9 (1.2%) a complete normalization from definite CAN [ ]. Younger age, HbA1c and body weight reduction, the absence of micro/macroalbuminuria, and shorter duration of diabetes were associated with CAN recovery.
The ADDITION-Denmark study reported also that neuropathic symptoms progressed in 23% and regressed in 26% of subjects with type 2 diabetes after 13 years [ ]. In the German Diabetes Study, including 179 and 291 patients with type 1 and type 2 diabetes with a duration <1 year, DPN was present at baseline in 8.1% and 13.3% of the type 1 and type 2 diabetes groups, respectively, and at the follow up of 5 years, stabilization, progression, and regression were observed. In particular, regarding the diagnosis of confirmed DPN, in type 1 diabetes 2.6%, 10.3%, and 5.8% remained abnormal, regressed to normal, and progressed to abnormal, respectively, and in type 2 diabetes, the figures were 7.8%, 6.5%, and 10.4% [ ]. Thus, not only the neuropathic symptoms but also the diagnosis of DPN, the neuropathic signs as well as structural and functional peripheral nerve measures could both progress and regress in type 2 diabetes and to a minor degree also in type 1 diabetes, while no predictors of the course of nerve function tests were identified [ ].
These recent findings of a slowing of neuropathy progression may be the consequence of an overall improvement in the glucose and cardiovascular risk factor control in these diabetic cohorts. The documentation of even regression of definite DPN and CAN in a few subjects challenges the idea of irreversibility of advanced stages of CAN and DPN and requires further evaluation to exclude, at least for CAN, the role of a limited ability of CARTs to classify CAN stages. These new findings, however, support a possible beneficial effect on DPN and CAN progression of an early diagnosis and intervention in type 2 diabetes.
Lifestyle intervention
Clinical and preclinical studies show the presence of DPN in prediabetes and metabolic syndrome and provide the experimental evidence of a detrimental effect on neurons of high-fat diet and the restoring action of lifestyle modifications that is confirmed by a limited number of clinical trials [ , ].
Physical exercise and DPN . Physical inactivity was found associated with the presence of DPN and painful DPN. Few studies have explored the beneficial effect of physical activity on DPN ( Table 9.4 ) [ , ].