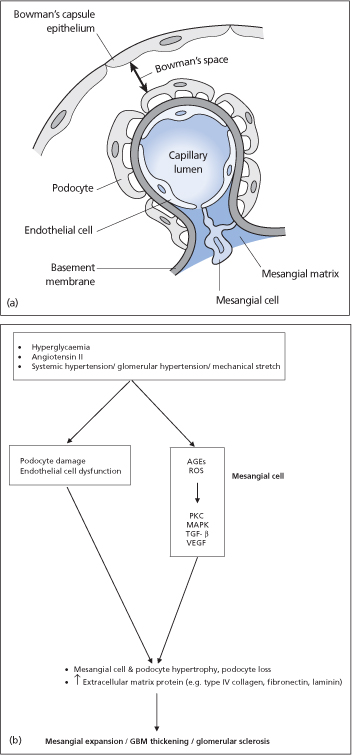
Hyperglycaemia results in increased reactive oxygen species (by increased activation of mitochondrial electron transport) and AGEs (generated by non-enzymatic combination of the excess glucose with amino acids in proteins). These in turn activate a number of signalling pathways involving protein kinase C, mitogen-activated protein kinase (MAPK) and transforming growth factor-beta (TGF-β), resulting in the accumulation of extracellular matrix proteins (e.g. collagen type IV and fibronectin) in the mesangial space, and ‘glomerulosclerosis’ (fibrosis in the renal glomeruli). Diabetes is associated with a reduced expression of renal bone morphogenic protein-7 (BMP-7), which appears to counter the profibrogenic actions of TGF-β.
Haemodynamic factors including systemic hypertension and glomerular hypertension contribute to the pathogenesis of diabetic nephropathy. Diabetes is associated with impaired renal autoregulation. As a result, increased systemic blood pressure does not produce the expected afferent arteriolar vasoconstriction and leads to intraglomerular hypertension. Glomerular hypertension, the resultant mesangial cell stretch and angiotensin II also stimulate glomerulosclerosis.
Other factors that may play a role in the development of diabetic nephropathy include increased plasma prorenin activity (which activates MAPK), increased expression of heparanase (causing a loss of negatively charged heparan sulfates and increased glomerular basement membrane permeability to albumin) and a reduced expression of renal nephrin (a transmembrane protein expressed by podocytes).
In addition to mesangial cells, glomerular endothelial cells, podocytes and tubular epithelial cells are also targets of hyperglycaemic injury. There is substantial crosstalk between endothelial cells, podocytes and mesangial cells. Endothelial dysfunction and podocyte damage may result in diabetic glomerulosclerosis.
The likelihood of developing diabetic nephropathy is markedly increased in patients with a diabetic sibling or parent who has diabetic nephropathy. A number of factors, such as increasing age, race, obesity, smoking and oral contraceptive use, may increase the risk of developing diabetic nephropathy.
Epidemiology
Diabetic nephropathy is the most common cause of end-stage renal disease in the UK and the USA.
Type 1 diabetes
After 10 years of type 1 diabetes, 20–30% of patients will have microalbuminuria. Less than 50% of patients with microalbuminuria will progress to proteinuria over an average period of 5–10 years. In total, 50% of patients with proteinuria reach end-stage renal disease after 10 years. Those patients who have no proteinuria after 20–25 years have a risk of developing overt renal disease of only about 1% per year.
Microalbuminuria may regress or remain stable with improved glycaemic and blood pressure control and the use of angiotensin-converting enzyme (ACE) inhibitors.
Type 2 diabetes
At 10 years following diagnosis, 25% of patients have microalbuminuria. The time from the onset of diabetes to proteinuria (around 15 years) and from the time from onset of proteinuria to end-stage renal disease (around 10 years) is similar in type 1 and type 2 disease.
Figure 38.2 (a) Glomerular cells. (b) Simplified model of pathogenesis of diabetic nephropathy. AGEs, advanced glycosylation end-products; GBM, glomerular basement membrane; MAPK, mitogen-activated protein kinases; ROS, reactive oxygen species; PKC, protein kinase C; TGF-β, transforming growth factor-β VEGF, vascular endothelial growth factor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree