Introduction
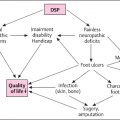
Diabetic foot disease (DFD) is a major global burden for patients and health care systems and is one of the most serious complications of diabetes mellitus . It encompasses infection, ulceration, and osseous destruction of the foot of a person with diabetes. Frequently accompanied with peripheral arterial disease (PAD), and neuropathy, which play a central role in the disease .
The International Working Group on the Diabetic Foot (IWGDF) publishes international, multidisciplinary, evidence-based guidelines to inform health professionals all over the world on the prevention and management of DFD .
DFD encompasses diabetic peripheral neuropathy (DPN), foot ulceration, Charcot neuroarthropathy (CN), peripheral vascular disease (PVD), which is a major etiological factor in diabetic foot lesions as neuroischemic ulcers present a major challenge in management. Infection, ulceration, or destruction of tissues of the foot of a person with currently or previously diagnosed diabetes mellitus, usually accompanied by neuropathy and/or PAD in the lower extremity.
While the prevalence of DFD varies in different regions of the world, the pathways for ulceration are similar in most patients. A holistic patient approach is recommended to identify the epidemiology of DFD to successfully treat and implement a suitable management plan .
Epidemiology of diabetic foot problems
Every hour, in England, someone older than 50 years of age has a foot amputation (below the ankle) due to DFD, and every 2 hours someone loses their whole leg (above or below the knee). The current UK annual cost of lower limb ulcer care is £4bn and predicted to rise to £7bn by 2022 . The burden on the NHS of patients with foot and leg ulcers is greater than obesity. One in three individuals with neuropathic DFD have a lower extremity amputation, and one in six have an early demise . The incidence of neuropathic foot ulcers is associated with more than seven times increase risk of mortality, compared to those who do not develop a foot ulcer, despite similar age and duration of diabetes .
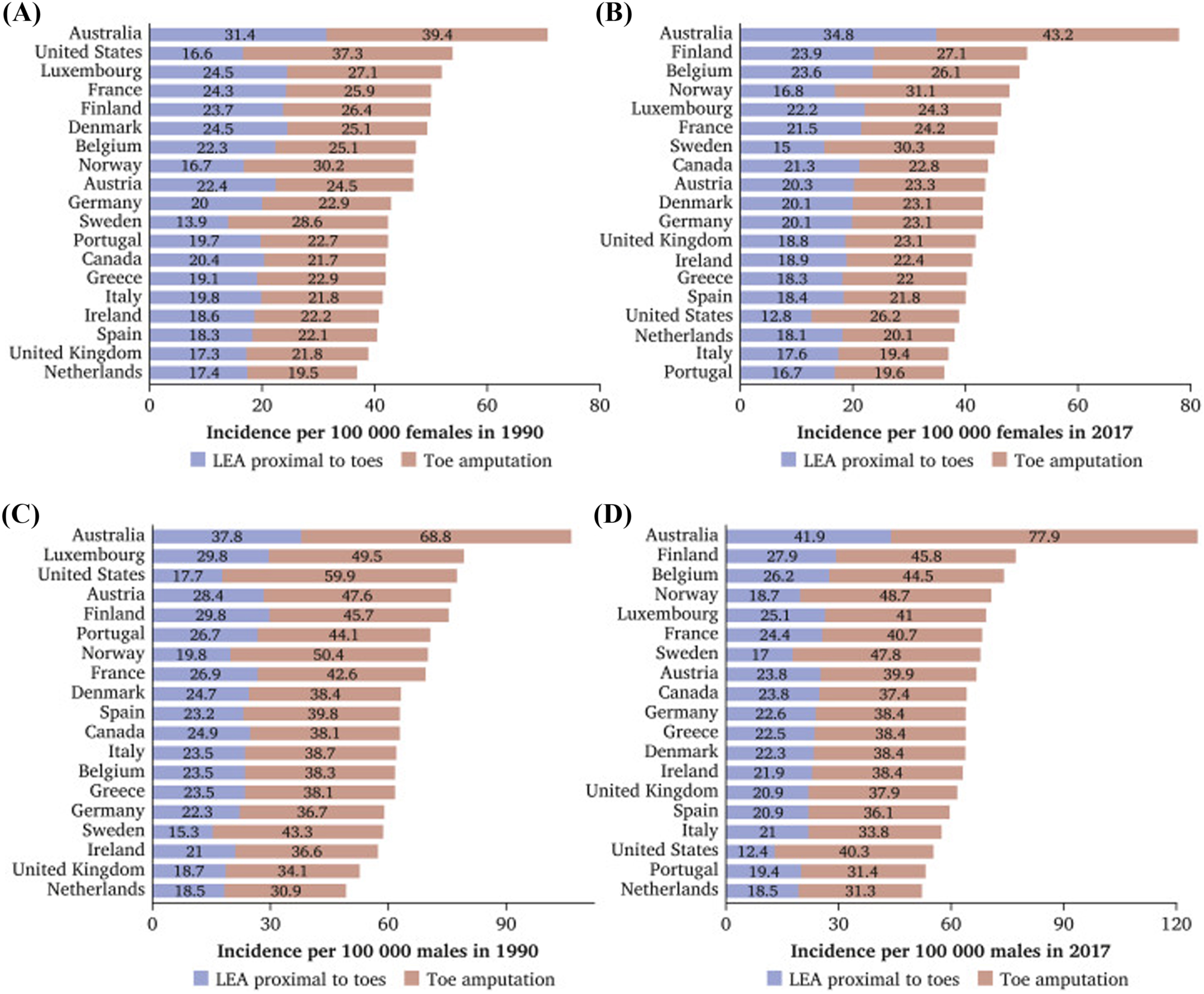
Annual incidence and prevalence of foot ulceration and amputation in the United Kingdom is not certain and is heavily debated, due to anomalies with how amputations are categorized, and the methodology used in the studies that report on incidence and prevalence. The range of rates of foot LEA in the diabetic population ranges from 1.2 to 362.9 per 100,000. The National Diabetes Foot Care Audit (NDFA) reports that the number of ulcer episodes submitted to the audit increased by 57% between 2016–17 and 2017–18 ( Fig. 14.1 ).
Risk assessment
Lack of symptoms and evidence of diabetic foot ulceration (DFU) do not exclude DFD. There is no evidence on the effect of screening for preventing DFU or its frequency, it is based on expert opinion, since there is no published evidence to support the required intervals. Implementing community diabetic foot screening is reported to cause greater good than harm . National Institute for Clinical Excellence Guidelines Ng19 set out the current UK guidelines for the prevention, management, and treatment of DFD, however the guidelines are focused on infection with limited guidance on PAD and foot ischemia detection, or diagnostic assessments relating to DFD .
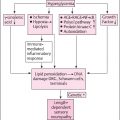
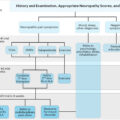
It is crucial to identify an “at risk foot” by carrying out an initial assessment and proceeding to in-depth diagnostics based upon the findings. Thorough history taking including diabetes diagnosis, comorbidities, current medication, previous ulcer/lower extremity amputation, claudication, loss of protective sensation (LOPS), foot deformity, vascular status, neuropathy status, should be identified. The IWGDF 2019 Risk Stratification System can be used to categorize risk ( Table 14.1 ).
| Category | Ulcer risk | Characteristics | Frequency |
|---|---|---|---|
| 0 | Very low | No LOPS and no PAD | Once a year |
| 1 | Low | LOPS or PAD | Once every 6–12 months |
| 2 | Moderate | LOPS+PAD, or LOPS+foot deformity, or PAD+foot deformity | Once every 3–6 months |
| 3 | High | LOPS or PAD, and one or more of the following:
| Once every 1–3 months |
Diabetic peripheral neuropathy
The yearly incidence of distal symmetric polyneuropathy in patients with diabetes is approximately 2%, and the lifetime incidence of neuropathy has been estimated to be 37%–45% for patients with type 2 diabetes and 54%–59% for patients with type 1 diabetes . Studies of nerve conduction tests performed at the time of diabetes diagnosis demonstrate that neuropathy is already present in 10%–18% of patients and subclinical neuropathy is also present . It is one of the most common diabetic complications and leading cause of disability, foot ulceration, and ultimately amputation . It is estimated to be present in at least 50% of people diagnosed with diabetes. The incidence of DPN in the diabetic population is associated with other potentially modifiable cardiovascular risk factors, including a raised triglyceride level, hypertension, obesity, and smoking .
It is defined as asymmetrical and length-dependent sensorimotor polyneuropathy, attributed to metabolic and micro vessel alterations due to long-standing hyperglycemia and metabolic derangement . Sensorimotor peripheral neuropathy can be asymptomatic, painful, painless, or both. The characteristic clinical manifestation of distal symmetrical sensorimotor peripheral neuropathy is a feeling of burning, tingling, electric, sharp, and shooting pain or a numbness and heaviness. Patients can also be unaware they have DPN as they may be asymptomatic . It’s reported that 80% of the DPN patients have depression and anxiety disorders, due to the ongoing symptoms of DPN . Autonomic dysfunction in the diabetic lower extremity can manifest as anhidrosis, and commonly callus formation will occur on weight-bearing areas of the foot .
The presence of moderately and severely increased albuminuria, that is, UACR of ≥30 mg/g, is a strong predictor of DPN . Long-term glycemic variability is also associated with diabetics that have DPN. Further studies are required to conclude albuminuria or glycemic variability for identifying individuals at increased risk for DPN . However, DPN is a culmination of a complex interaction of several causatively linked pathophysiological processes, many of which are not fully understood. Hyperglycemia and duration of diabetes have an important role in DPN .
It is important to determine risk factors and control them at an early stage to prevent serious consequences, such as foot ulcers, gangrene, and amputation. Current studies suggest that the risk factors include the duration of diabetes, age, increased HbA1c, diabetic retinopathy, smoking, and a high body mass index. Appropriate interventions to address risk factors can reduce ulcers by 60% and amputations by 85% in those with high-risk diabetic neuropathy .
Neurological assessment
Nerve conduction studies are the gold standard for diagnosis of DPN, however are time consuming, costly, and impractical to implement in routine clinical care screening programs. Measures used to screen for DPN in clinical practice are rudimentary and do not detect the disease until the late stages of its development .
Using standardized clinical assessment scoring such as the Michigan Neuropathy Screening Instrument, the Toronto Clinical Neuropathy Score, and the UK neuropathy disability screening scores are subjective, and reliant on the clinician’s interpretation .
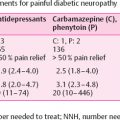
Semmes Weinstein Monofilament Examination (10-g monofilament) is currently the gold standard for first-line screening of DPN in the feet. Other neurological screening tools include the Ipswich Touch Test, whereby the clinician uses their index finger to apply light touch to the tips of the first, third, and fifth toes. Neuropathy is identified when detection of sensation fails at two or more sites (out of the total six) . Stimulation tests are also used such as biothesiometer and the vibration perception threshold testing with the Vibratip, a tuning fork, or automated devices such as the Vibration Sensory Analyser VSA-3000, or Neurometor . These assessments are reliant on patients’ subjective response. They are mainly used to determine loss of protective foot sensation and risk of ulceration .
Nerve condition studies only assess large nerve fibers. DPN involves both small and large nerve fibers, with small nerve fiber involvement occurring at the early stage of the disease. Small nerve fibers constitute 80%–91% of peripheral nerve fibers, which control pain perception and autonomic function. There has been development for the early detection of DPN using devices such as DPNCheck that assesses the sural nerve in 3 minutes, with 95% sensitivity and a 71% specificity (DPNCheckTM, 2020), and Neuropad that is a 10-minute test that measures sweat production. While Neuropad reported to be highly sensitivity for small fiber neuropathy, it has low specificity . These devices are in their infancy of experimental use and have high heterogeneity and participant selection bias cannot be discounted . Intraepidermal nerve fiber density measurement from lower limb skin biopsy is considered the gold standard for the diagnosis of small fiber neuropathy but is invasive and not suitable for DPN screening ( Table 14.2 ).
| Inspection |
|---|
| Dermatologic |
|
| Musculoskeletal |
|
| Neurological assessment |
| 10-g monofilament+1 of the following 4 |
|
| Vascular assessment |
|
Five simple clinical tests are considered useful in the diagnosis of LOPS in the diabetic foot . Any of the five tests could be used by clinicians to identify LOPS. Two of these tests should be regularly performed during screening assessments which are usually the 10-g monofilament and one other test .
One or more abnormal tests would suggest LOPS, while at least two normal tests (and no abnormal test) would rule out LOPS . The vibration assessment using a biothesiometer or similar instrument is a useful tool in the assessment of LOPS ( Table 14.3 ).
| 10-g monofilament at 4 sites on each foot + 1 of the following: |
| Vibration using 128 Hz tuning fork |
| Pinprick sensation |
| Ankle reflexes |
| Vibration perception threshold |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree