Autonomic neuropathy
Diabetes, like aging, precipitates a cascade of secondary symptoms: sleep and GI disturbance, dizziness or lightheadedness, secondary hypertension, urogenital dysfunction, higher risk of cardiovascular disease, and more. This is a recurring theme and may help to lend more insight to pathogenesis, identification, diagnosis, and treatment of the effects of diabetes on the P&S. P&S decline, regardless of rate, seems to follow the same progression whether it is caused by healthy aging or chronic disease.
As discussed and referenced in Chapter 10 , poor P&S results have been correlated with poor outcomes, and normal P&S results have been correlated with healthy outcomes. For example, orthostatic hypotension (OH) is common in diabetes. OH is a failure of the sympathetics, known as sympathetic withdrawal (SW). 1
1 Sympathetic Withdrawal (SW, which we will discuss later) is the neurological basis of orthostatic disorders. It is the abnormal Sympathetic response to postural change where the sympathetics decrease rather than increase.
SW is not a structural deficit; it is a functional deficit. Identifying this difference facilitates diagnosis and therapy.In addition to lightheadedness (or dizziness often from orthostasis), the common comorbidities that are associated with diabetes mellitus include:
- •
Hypertension secondary to the diabetes, and possibly secondary to SW;
- •
Upper and lower GI upset, possibly due to parasympathetic dysfunction;
- •
Sleep disturbances, possibly due to either or both P&S dysfunction;
- •
Genitourinary dysfunction, possibly due to either or both P&S dysfunction;
- •
Cardiovascular diseases, possibly due to either or both P&S dysfunction; and
- •
Renal disorder, possibly due to either or both P&S dysfunction.
The effect of autonomically active therapy (e.g., beta-blockers, antihypertensives, antidepressants) is to help establish and maintain normal SB. Therapy should be titrated to normalizing SB for the individual patient to relieve the comorbidities. Then the patient becomes more stable, and the physician may be more aggressive toward the primary disease.
Earlier detection and earlier intervention
The above short list of common comorbidities may be substantiated by the resting P&S imbalances indicated in the curves presented in Fig. 10.2 of Chapter 10 ; however, they may have origins long before these more traditional definitions of autonomic neuropathy. As mentioned earlier, orthostatic hypotension (OH), or more generally, orthostatic dysfunction (including OH, orthostatic intolerance, or OI, and postural orthostatic tachycardia syndrome, or POTS), is arguably the most debilitating symptom of diabetes , and possibly P&S dysfunction in general. Again, the autonomic basis underlying orthostatic dysfunction is SW. This dysfunction, documented during a head-up, postural change (e.g., standing), is a dysfunctional challenge response (rather than dysfunctional resting response). While orthostatic dysfunction (e.g., OH) may mark the beginning of DAN or CAN, SW often occurs long before DAN or CAN. Recent clinical experience suggests that correcting SW, and any accompanying challenge autonomic dysfunction, may help to slow the progression of resting P&S dysfunction (i.e., DAN or CAN). SW and other postural-change P&S dysfunctions are detailed in Chapter 10 .
SW and the associated orthostatic dysfunction may be treated with high dose r-alpha-lipoic acid (600 mg, tid, time release recommended to minimize acid effect on stomach) or low-dose oral vasoactive therapy (i.e., midodrine, 2.5 mg, tid, titrated slowly, assuming no supine hypertension or resting BP above 160/90 mmHg) .
As discussed in Chapter 10 , small fiber neuropathy is also a precursor to autonomic neuropathy. The small nerve fibers include autonomic nerve fibers, and both are often comorbid. Small fiber disorder is mostly associated with generalized pain disorders, and it may be an even earlier indicator of autonomic dysfunction. The small sympathetic fibers, which uniquely control the vasculature, may be affected by small fiber disorder. This Sympathetic affect may underlie poor peripheral circulation leading to poor wound healing and dry skin, which are also early signs of diabetes. Small fiber disease may be treated with OTC methyl-folate or, if prescription grade is needed, metanx .
Disease initiation and progression of neuropathy
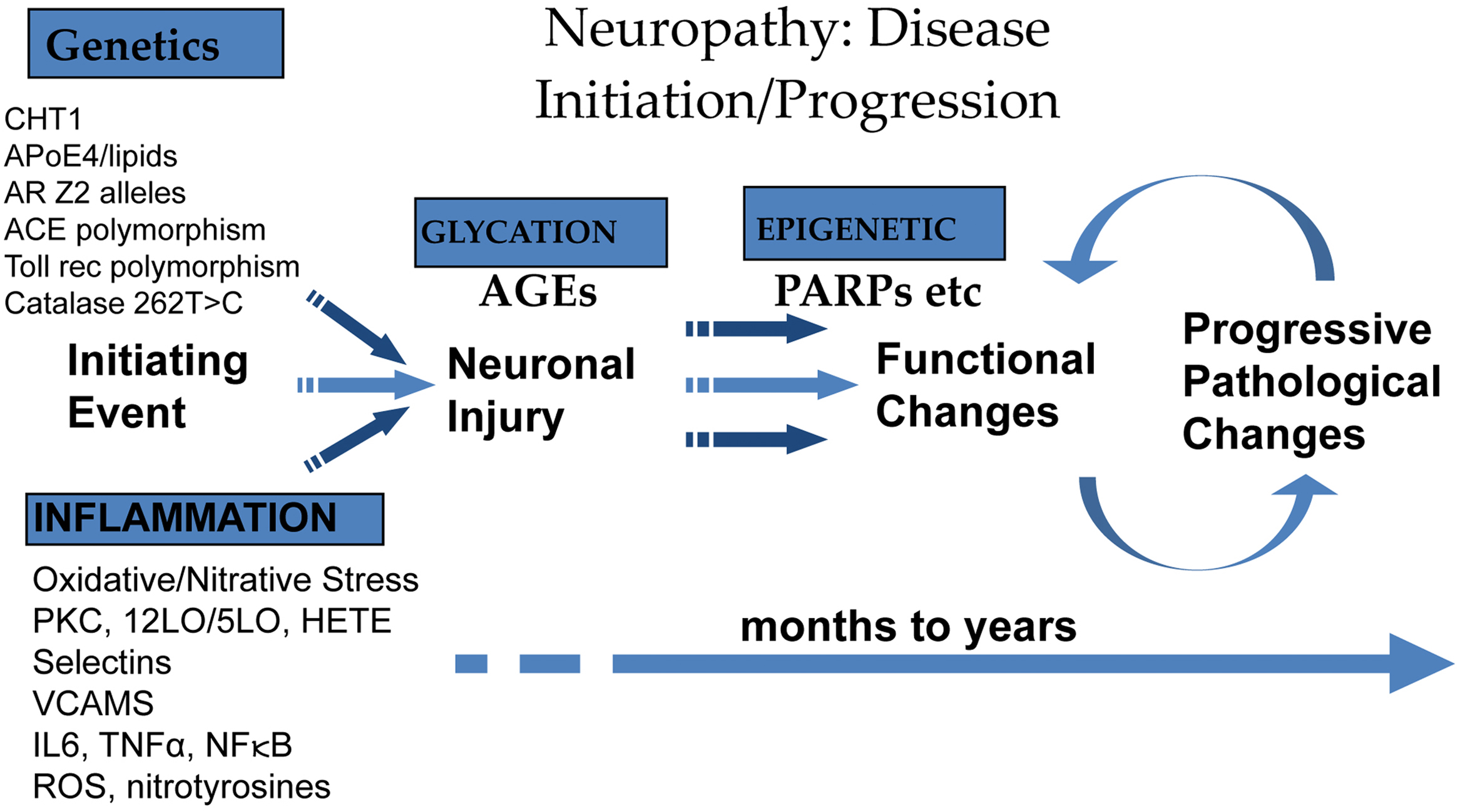
It is well known that in diabetes, causative factors include persistent hyperglycemia, microvascular insufficiency, oxidative and nitrosative stress, defective neurotropism, and autoimmune-mediated nerve destruction . DAN is a heterogeneous group of conditions with widely varying pathology, suggesting differences in pathogenic mechanisms for the different clinical syndromes . Recognition of the clinical homolog of these pathologic processes is the first step in achieving the appropriate form of intervention.
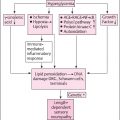
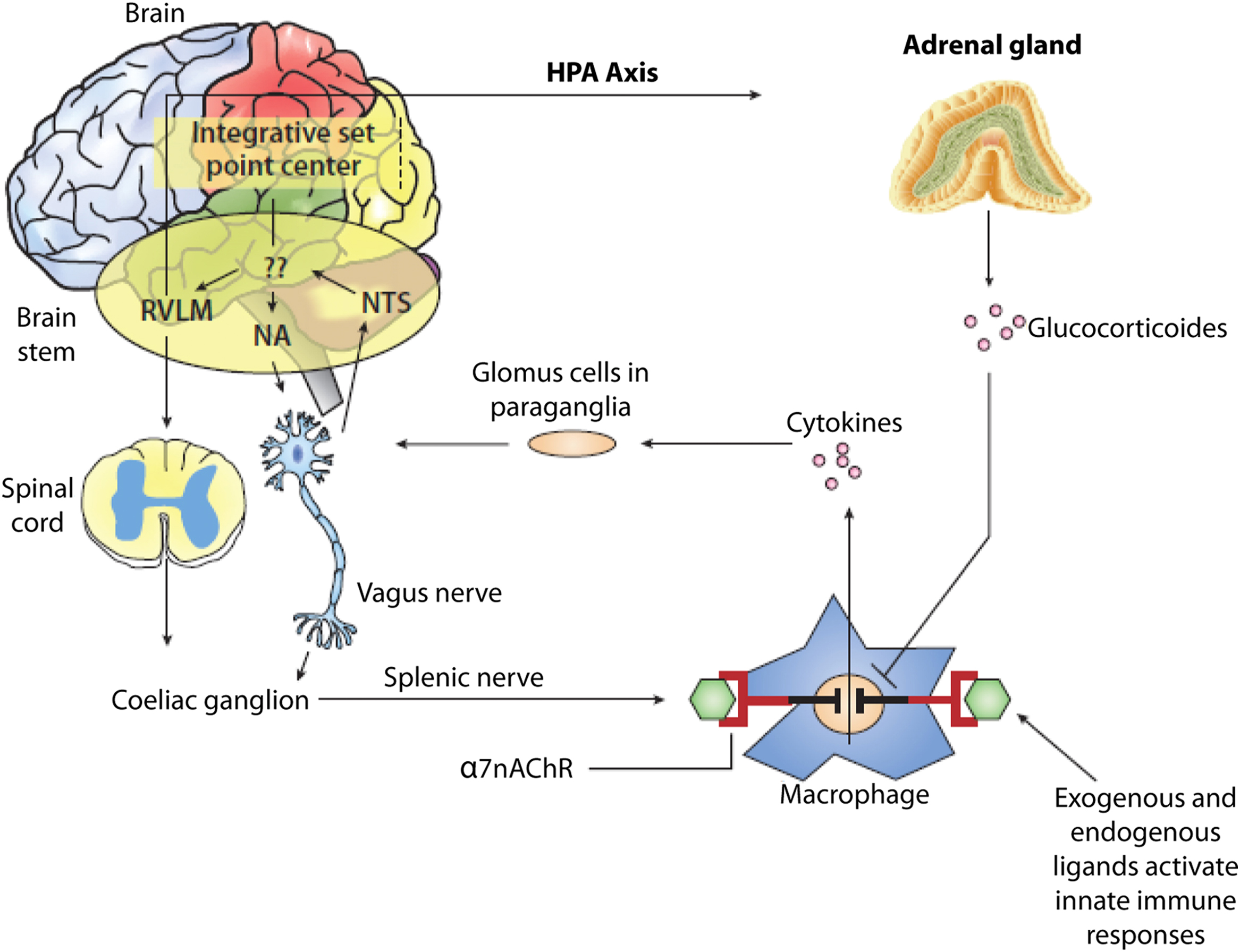
The link between autonomic and inflammatory pathways and their response to endogenous or exogenous stressors is the sympathetic nervous system. Prolonged or excessive sympathetic activation is abnormal and leads to increased morbidity and mortality risk. Psychosocial and physical stresses, as well as the stresses of disease, disorder, or injury, may all lead to prolonged or excessive sympathetic activation. Prolonged or excessive sympathetic activation results in increased hepatic gluconeogenesis, a rise in circulating free fatty acids, resistance to the action of insulin, and increases in markers of inflammation and hypercoagulation. All of these may be restored toward normal by treatment to restore P&S balance which may reverse the metabolic abnormalities simply by enhancing parasympathetic dominance to normalize SB. There are simple treatments and therapies to improve SB . In contrast, failure to identify loss of parasympathetic integrity is accompanied by potentially dire consequences as witnessed by the 22% increase in sudden death in the ACCORD study with intensification of therapy . The association between inflammation (with activation of inflammatory cytokines such as IL-6 and leptin), oxidative stress, and abnormalities in SB is depicted in Fig. 19.1 . Autonomic dysfunction has been shown to be a predictor of cardiovascular risk and sudden death. Activation of the efferent arm of the reflex arc (through the administration of an acetylcholine receptor agonist, see Fig. 19.2 ) causes a decrease in proinflammatory cytokine production, and a reduction in disease severity .
Oxidative stress
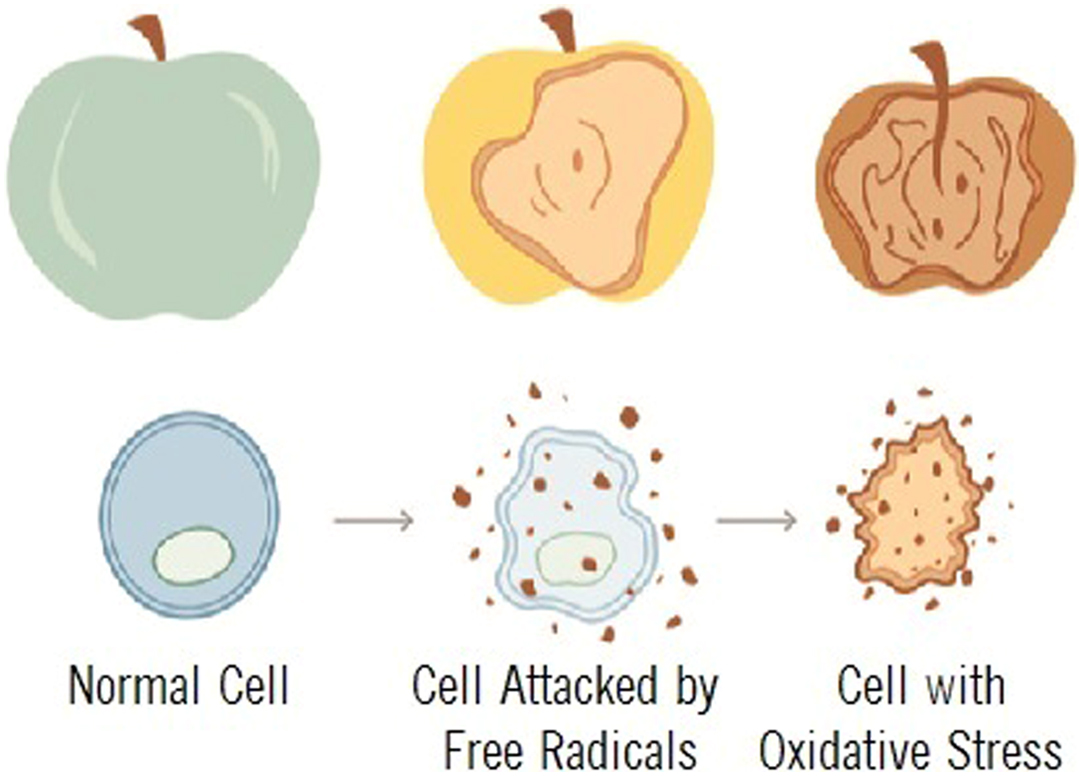
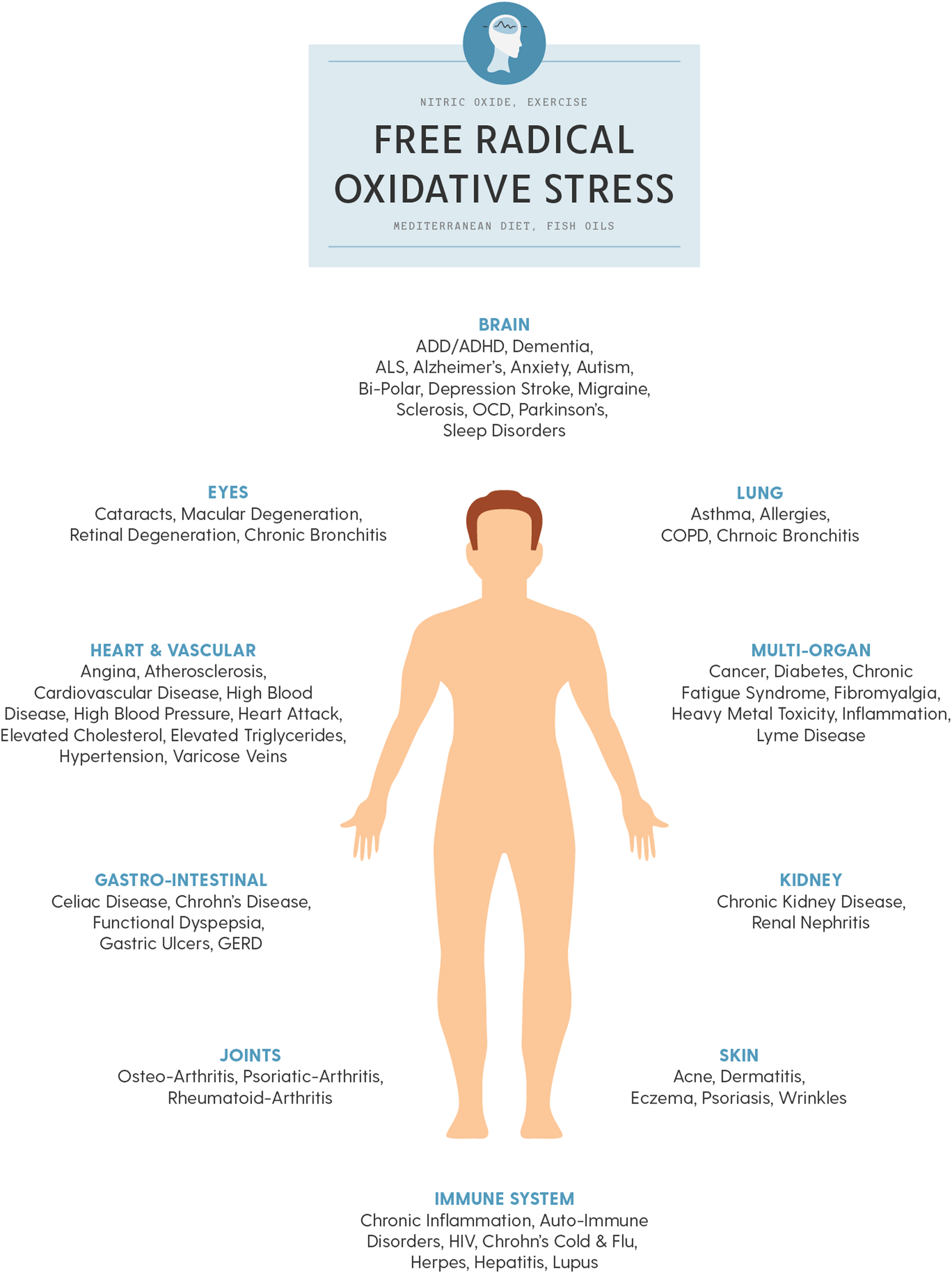
All-cause mortality is greater than double for those diagnosed with type 2 diabetes and DAN . Oxidative stress is described as the mechanism related to autonomic injury found with DAN , which often deteriorates to CAN which, untreated, leads to sudden cardiac death . Oxidative stress is stress at the cellular level. The oxidation of fruit, like the apples in the insert below, demonstrates how oxidative stress breaks down cells, causing premature aging, fatigue, and disease. Oxidative stress is mediated through free radicals and reactive oxygen species (ROS) and reactive nitrogen species (RNS, ROS, and RNS collectively are known as RONS). Oxidative stress targets cellular membranes, DNA, and especially mitochondria. In fact, mitochondria are the largest producers of ROS in the body. While most ROS produced by the mitochondria are collected by the immune system and used as a first-line defense against invading pathogens, increased oxidative stress overwhelms the system and damages mitochondria, reducing energy (ATP) production and leading to fatigue, especially when attempting to be active, mentally as well as physically. Over the short term, as in responding to a cold or the flu, fatigue is helpful in that it promotes rest and sleep to help the healing process. However, with long-term oxidative stress, mitochondria (among other organelles) are severely damaged, and persistent fatigue or chronic fatigue is one of the resulting symptoms . Fig. 19.3 presents the systemic effects of oxidative stress, highlighting the many complications of diabetes.
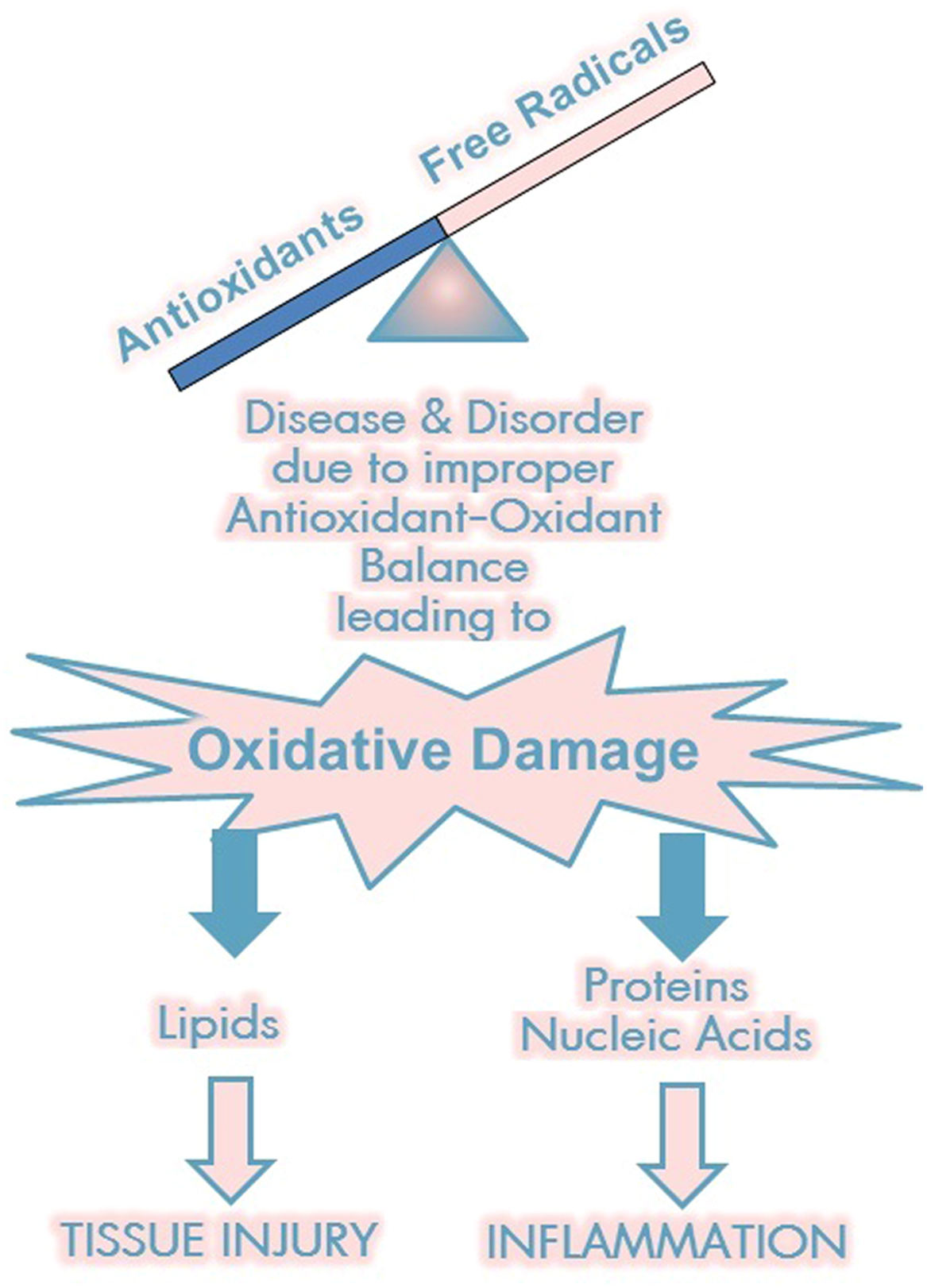
Oxidative damage affects proteins, nucleic acids, and lipids; its effects on proteins and nucleic acids lead to increased inflammation and its effects on lipids exacerbate to nerve injury. Hyperglycemia reduces antioxidant activity, increasing inflammation, lipid peroxidation, the production of AGEs, glycated proteins, and ketones, altering genetic transcription activation, damaging energy production, and increasing apoptosis and cell death. All of these affects are also involved in aging. Oxidative stress can feed on itself, for example, through lipid peroxidation and increased inflammatory cytokines .
Cardiovascular risk factors and vascular disease are associated with increased levels of oxidative stress. Carvedilol possesses antioxidant effects, which may explain its preference in diabetes . Traditional risk factors for circulatory diseases have been identified, and excellent pharmacological and lifestyle measures to improve these risk factors have been developed. Traditional risk factors include diabetes, high LDL cholesterol, low HDL cholesterol, family history of premature cardiovascular disease, 2
2 This is considered a nonmodifiable risk factor.
male gender, hypertension, being sedentary, aging, obesity, and tobacco . Nontraditional risk factors include psychosocial stress, inflammation, endothelial dysfunction, kidney disease, and poor brachial index measurements . Atherosclerosis is a common circulatory disease and is highly associated with endothelial dysfunction. A vicious cycle between oxidative stress and oxidative stress-induced atherosclerosis leads to the development and progression of atherosclerosis .Sleep apnea is a cause of oxidative stress, compounding the stress, leading to or exacerbating associated conditions and comorbidities, such as sympathetic overactivation, persistent obesity, hypertension, hyperlipidemia, and diabetes. Of course, these conditions also cause oxidative stress, exacerbating the condition and perpetuating this vicious cycle, eventually involving inflammatory and immune cell activation and cardiovascular disease. Sleep apnea is associated with elevated sympathetic activity that may be, partially, ameliorated by CPAP treatment . It is important to know, however, that CPAP is not a sympatholytic. It only has an indirect effect on the sympathetics. It relives the stress of not sleeping well, but does not actively, directly, reduce sympathetic excess. If there are other reasons for sympathetic excess (e.g., diabetes, hypertension, or obesity), CPAP alone is typically not sufficient to relieve sympathetic excess.
Considering metabolic syndrome as a precursor to type 2 diabetes, significant aldehyde production through lipid peroxidation is tightly linked to high-fat diet and obesity. Increased oxidative stress in accumulated fat is an important pathogenic mechanism of obesity-associated metabolic syndrome . Chronic, low-grade inflammation of white adipose tissue is a hallmark of obesity and a major contributor to oxidative stress and lipid peroxidation . In the expanding adipose tissue, hypertrophied adipocytes contribute to the inflammation by upregulating the expression and release of proinflammatory cytokines. Aldehydes also decrease the expression of the antiinflammatory, insulin-sensitizing hormone, adiponectin , therefore linking lipid peroxidation by-products and chronic inflammation .
With diabetes, aldehyde concentration is increased in several tissues, including the pancreas, liver, brain, and heart. Oxidative stress, through ROS, can positively and negatively regulate insulin signaling, depending on time, dose, mode, and free radical used . However, prolonged oxidative stress impairs insulin signaling and glucose uptake. Aldehyde inhibition of mitochondrial aconitase (a hydroxyl radical) enables the generation of precursors of fatty acid synthesis . However, data suggest that accumulation of lipid aldehydes and fat deposition could be mutually inductive, leading to a vicious cycle promoting fat accretion . Supplemental glutathione helps this, as glutathione levels in diabetic patients may be depleted. An increase in local glutathione may prevent the deleterious effects of aldehyde on ROS production , and that dysfunction of glutathione S-transferase, a major enzyme for aldehyde detoxification, leads to oxidative stress .
In diabetes, a contributor to oxidative stress is an excess of advanced glycation end-products (AGEs). AGEs are the result of nonenzymatic additions of glucose or other saccharides to proteins, lipids, and nucleotides. AGE generation leads to intra- and extracellular protein cross-linking and protein aggregation, which alters intracellular signaling and gene expression, releases proinflammatory molecules, and results in an increased production of ROS . Compounds including vitamins B 6 and B 12 with the antioxidant r-ALA have helped reduce the production of ROS and the subsequent effects of AGE-induced oxidative stress.
Protein kinase C (PKC) activation is a critical step in the pathway to diabetic microvascular complications leading to cardiovascular disease . It is activated by both hyperglycemia and disordered fatty-acid metabolism resulting in increased production of vasoconstrictive, angiogenic, and chemotactic cytokines including transforming growth factor β (TGF-β), VEGF, endothelin-1, and intercellular adhesion molecules .
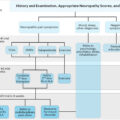
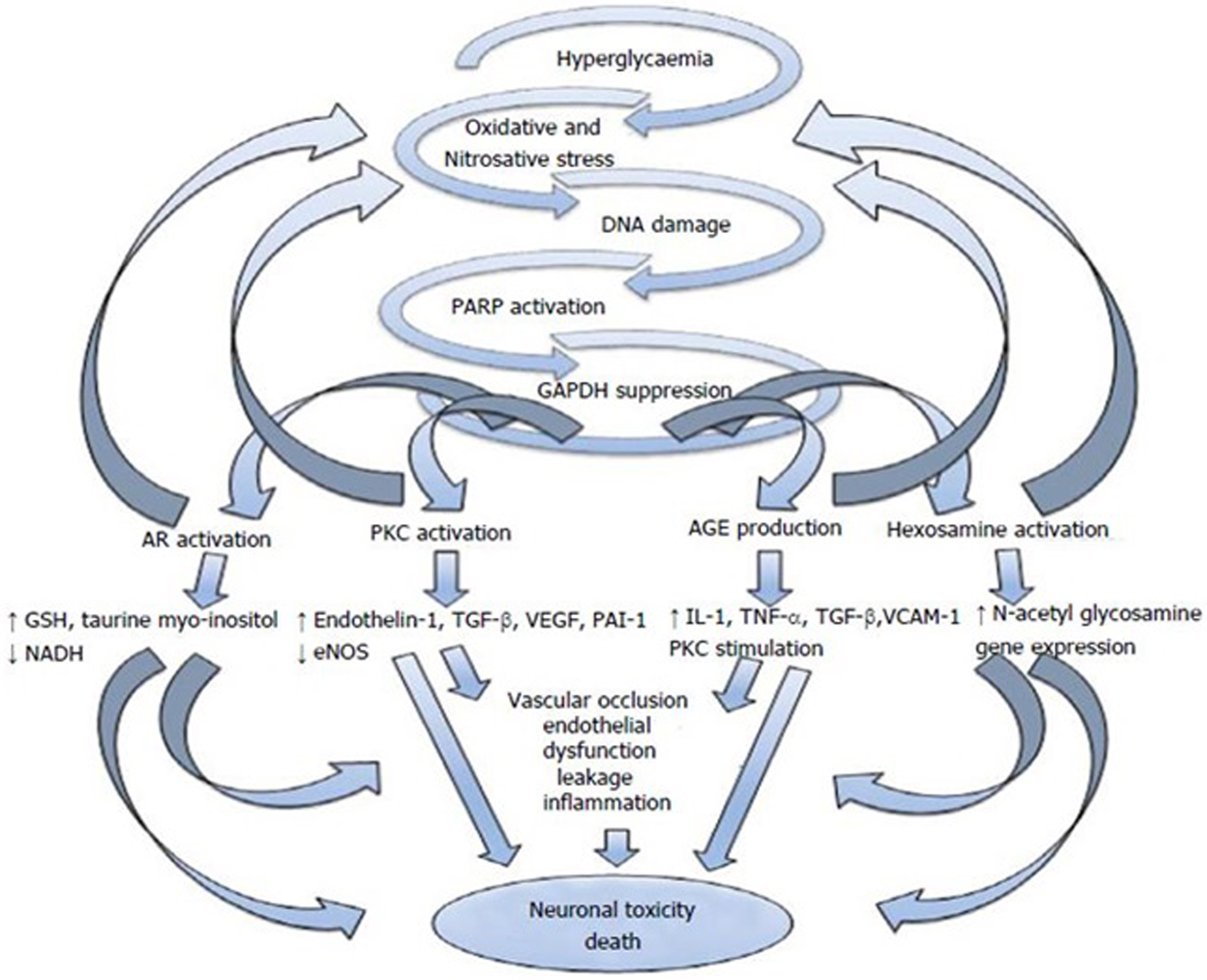
There is evidence that oxidative stress leads to a deficiency of growth factors in diabetes and contributes to clinical perturbations in small-fiber function and, in the pancreas, a pancreatic cytokine . As shown in Fig. 19.4 , increased oxidative stress effects four pathways that contribute to glucose toxicity . These different pathways can in return exacerbate oxidative stress and can induce changes in gene expression, transcription factors, diverse cellular products disrupting several cellular functions and the communication between the cell and the surrounding matrix, all of which leads to neuronal dysfunction and death . These pathways also result in impaired microvascular regulation and endothelial dysfunction by different pathways . Diabetes affects the enteric nervous system (considered as a part of the parasympathetic nervous system) and thereby GI manifestations of diabetes .
Patients who have type 2 diabetes are two to four times more likely to develop heart failure than those without diabetes. However, heart failure is also a risk factor for diabetes. At healthy levels, ROS are used to regulate multiple vascular cell functions, including endothelial cell and smooth muscle cell growth proliferation, angiogenesis, and vascular tone . Heart failure is categorized by activation of the sympathetic and renin-angiotensin systems. These neuroendocrine activations are associated with oxidative stress in the myocardium and vasculature. In heart failure, oxidative stress occurs in the myocardium and plasma and correlates with left ventricular dysfunction . Excessive levels of ROS (due to diabetes or heart failure) adversely affect the disposition of myocardial calcium, causing arrhythmia and abnormal cardiac remodeling and left ventricular hypertrophy, apoptosis, and necrosis, and they cause uncoupled nitric oxide synthase (NOS). Angiotensin-converting enzymes and angiotensin receptor blockers exert their beneficial effects in part through antioxidative mechanisms by blocking ROS production at the enzymatic source, the mitochondria.
The omega-3 fatty acid, eicosapentaenoic acid (EPA), is widely used to fill the “Statin Gap” in diabetics and others with cardiovascular disease due to endothelial dysfunction. This is due to EPA’s healthful effects on lipoprotein metabolism, platelet function, cytokine production, clotting, fibrinolysis, and inflammatory factors. Diabetes and obesity have similarities in that fatty acid uptake and fatty acid oxidation are increased as levels of intramyocardial circulating triglycerides, circulating free fatty acids, glucose uptake, and glucose oxidation are reduced . The ability of obese or diabetic hearts to use glucose results in reliance on fatty acid oxidation and reduces metabolic flexibility. Free fatty acids increase ROS production in mitochondria, which increases mitochondrial dysfunction leading to (among other dysfunctions) increased myocardial ROS emission . Hyperglycemia induces the mitochondria to produce excess superoxide (O2-, a type of ROS) from increased flux in the electron transport chain. This is a major cause of the clinical consequences associated with diabetes and obesity . In this way, hyperglycemia activates four major pathways in the pathogenesis of cardiovascular complications of diabetes and obesity including (1) a path that increases production of advanced glycation end products (AGEs), (2) the polyol pathway, (3) the hexosamine pathway, and (4) the protein kinase C-dependent signal transduction .
Treating diabetic autonomic dysfunction and neuropathy
Pharmacology and “Functional Medicine” are complimentary. Prescription medicines are needed to ensure the disease or disorder is stabilized and at least partially relieved, and then functional medicine (i.e., supplements and lifestyle modifications, including diet and exercise) completes the therapy and helps to establish and maintain wellness. Traditional approaches should not be discounted but should incorporate complimentary (nontraditional) approaches as well. To this end, for example, two pharmaceuticals often prescribed to diabetics are metformin and carvedilol; both have antioxidant effects .
The P&S have been shown to be reflective of medication effects (both direct and indirect), as well as disease and aging effects. Many common medications are known autonomic agents and may be used off-label, typically in very low dose, to balance P&S activity both at rest and in response to challenge, for example:
- •
Sympatholytics, including: beta-1 (adrenergic) blockers, beta-2 adrenergic agonists (bronchodilators), angiotensin converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers, etc.:
- •
Carvedilol is both a beta-1 blocker and an alpha-adrenergic blocker;
- •
- •
Antihypertensives (including alpha-adrenergic antagonists);
- •
Anticholinergics with antidepressant affects, including very low dose antidepressants (e.g., tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors (SNRIs), and selective serotonin reuptake inhibitors (SSRIs)); and
- •
Oral vasoactive medications, including: alpha-1 (adrenergic) agonists (vasopressors, e.g., midodrine), droxidopa (northera), pyridostigmine (mestinon), and (rarely) pseudoephedrine.
Only midodrine and droxidopa are FDA approved for dysautonomia. They are approved specifically for NOH and help to relieve SW. Patient reactions to disease, therapy, medication, and dosing may be directly monitored and documented by P&S responses and used to further guide individualized therapy.
Of course, first treat diabetes according to standards to relieve the adverse effects of abnormal sugar and insulin levels on the nervous system and the rest of the body. Standard multifactorial treatment of hyperglycemia, lipids, BP, and other causal factors of diabetes may reduce abnormalities in autonomic function by up to 68%, especially when detected early. Autonomic dysfunction will still progress, albeit slower (perhaps), and dysautonomia, DAN, and then CAN will ultimately present. Additional P&S therapy for dysautonomia (including DAN and CAN) helps to further slow the progression of P&S dysfunction and continue to reduce morbidity and mortality risks. Frequent monitoring and earlier treatment of dysautonomia helps to delay even further the onset of DAN and CAN and helps to maintain a more normal quality of life. Treating DAN, CAN, or any dysautonomia first involves establishing and maintaining proper balance: normalized SB and normalized challenge responses . Disorders or symptoms that remain are then a result of end-organ dysfunction, including oxidative stress and inflammation . Restoring proper SB 3
3 SB is the measure of resting P&S balance.
and P&S 4
4 P&S balance refers to a challenge P&S balance.
balance is possible , for example:
- •
Resting SE(measured as high SB) may be treated with beta-blockers and antihypertensives, typically low doses unless complicated by known cardiovascular disease.
- •
Challenge SE (documented during either or both the Valsalva or stand challenge) is typically secondary and is often relieved by treating the underlying PE or SW.
- •
Resting PE (measured as low SB) or challenge PE (documented during any of the P&S challenges: deep breathing, Valsalva, or stand) may be treated with low dose anticholinergics (e.g., very low-dose antidepressants), or low-and-slow exercise:
- •
Challenge PE in response to deep breathing challenge may be confounded by pulmonary or upper respiratory disorder.
- •
SW may be treated with proper daily hydration (including fewer alcoholic, sugary, and caffeinated drinks and perhaps fewer diuretics) typically with electrolytes, oral vasopressors, or r-ALA. SB may be adjusted with agents that act on one or another arm of the ANS (see Fig. 19.5 ).

Pharmacological, supplement, or lifestyle modification manipulates P&S balance and leads to changes in the symptom complex and quality of life . This may include overmedication which will shift balance too far in the other direction (e.g., too much beta-blocker or antihypertensive may drive the sympathetics too low, enabling parasympathetic dominance, which may induce exercise intolerance and fatigue). Intervention studies have documented the protective and positive P&S effects of glycemic control in type 1 diabetes (e.g., DCCT ) and of multifactorial strategy aimed at lifestyle change with pharmacological correction of hyperglycemia, hypertension, dyslipidemia, and microalbuminuria . R-ALA is an example of functional medicine. Ziegler and Murray reported that r-ALA improves P&S function and reduces morbidity and mortality risk in patients diagnosed with diabetes. Note, several diabetes medications are listed as “bad drugs.” Remember, FDA drug approval is based on an approximate 80% effectiveness. The “bad drugs” are labeled so, based on the other 20%. P&S responses help to identify earlier when a patient is not responding to these (or any other) medications as expected and provides the documentation to switch to another agent, thereby, preventing the effects of “bad drugs.”
After establishing P&S balance , therapy may include treating any symptoms that remain. Take care to not overtreat and re-establish P&S imbalance. Also, it may take 3–6 months for remaining symptoms to be relieved organically. For example, should high BP remain once SW or PE are relieved, it may take approximately 3 months for the secondary SE to be relieved, then approximately 3 more months later, associated high BP will normalize . Of course, this assumes no atherosclerosis, or hardening of arteries, or other nonneurogenic causes of high BP. In addition to high BP, remaining symptoms may include resting tachycardia or exercise intolerance, atherosclerosis, or other cardiovascular diseases, nonneurogenic postural hypotension, nonneurogenic lightheadedness, dizziness (vestibular in origin), weakness, fatigue (perhaps largely due to oxidative stress), cardiogenic syncope, upper or lower nonneurogenic gastrointestinal symptoms, persistent inflammation, generalized pain, sudomotor dysfunction (including anhydrosis, heat intolerance, dry skin, or hyperhidrosis), vision affects, urogenital dysfunction (both male, including erectile dysfunction, or female, including vaginal dryness), other hormonal disorders, and impaired visceral sensations.
Continued low-and-slow or graded, supervised exercise often helps with many of these remaining symptoms. Exercise is arguably the most powerful of antioxidants, among its other beneficial properties. CoQ10 and other antioxidants in addition to r-ALA help to address any remaining effects of oxidative stress, atherosclerosis, and some of the other cardiovascular diseases. Supplemental oral vasoactives (e.g., fludrocortisone 5
5 A short course of Fludrocortisone (Florinef), prior to Midodrine, is recommended if resting BP is more than 160/90. Take care, over the long term, as fludrocortisone may induce fibrosis.
or pyridostigmine 66 Pyridostigmine (Mestinon) often causes diarrhea.
) may help to support midodrine or droxidopa. Carvedilol (with its additional antioxidant effects, rather than metoprolol 77 Carvedilol demonstrates a small but significant rebound in parasympathetic activity; metoprolol does not [].
) may be considered for tachycardia and some cardiovascular diseases. Methyl-folate (supplement) or metanx (prescription) may be considered for sudomotor dysfunction to help relieve the inflammation of small fiber disease and oftentimes heal the small fibers. Metanx has been shown to increase the number of nerve fibers, possibly reversing the damage done by diabetes, and is generally well tolerated. Desmopressin may be considered as adjunctive to fluids and electrolytes if the fluids (with electrolytes) are not staying in the veins, but rather is being spilled through the kidneys. The algorithm below provides an example of the therapy options for SW.