Autonomic neuropathy
A brief history
Autonomic Neuropathy is well established as a “crystal ball” for morbidity and mortality risks in patients diagnosed with Diabetes . Unfortunately, Autonomic Neuropathy is poorly understood and perceived to be untreatable . Yet significant morbidity and mortality risks may now be attributable to autonomic imbalance between the Parasympathetic and Sympathetic (P&S) nervous systems and their regulation of cardiovascular and respiratory function , indeed the function of all organs and virtually every cell in the human body. Heart rate alone , nor heart rate variability (HRV) alone , provides reliable diagnostic criteria for Autonomic Neuropathy. Diabetes, with its many factors and symptoms [e.g., high blood sugar, low insulin, obesity, overproduction of inflammatory cytokines, sleep apnea, high blood pressure (BP), oxidative stress, etc.], leads to, and exacerbates, P&S imbalance .
In Diabetes, these factors and symptoms are mostly mediated by the Sympathetic nervous system, causing or contributing to Sympathetic Excess (SE). This SE is typically the beginning of P&S imbalance in most patients diagnosed with Diabetes. SE will cause a Parasympathetic Excess (PE), as the still healthy P&S nervous systems attempt to reestablish proper balance. Eventually, prolonged SE prolongs PE, and leads to an accelerated aging of the Parasympathetic nervous system, which is the beginning of Autonomic Dysfunction and its progression to Autonomic Neuropathy. Granted, earlier and more aggressive treatment has become the norm for Diabetes, based on published standards, diagnosis and intervention is usually not early enough and oftentimes therapy is too much. Since the typical measures of Diabetes are Sympathetic in nature, and the drive is to normalize the symptoms of SE, many times the Sympathetics are unintentionally overmedicated, exacerbating the PE and further accelerating the onset of Autonomic Neuropathy. More information is needed to improve outcomes.
It has long been well established that restoring proper autonomic balance reduces morbidity and mortality, thereby improving outcomes . To do so, independent, simultaneous measurements of P&S activity is needed. Unfortunately, virtually all autonomic measures are measures of only total autonomic activity (which is a measure of the combined P&S effect), and the activity of the two autonomic branches is based on assumption and approximation or criteria that require prolonged suffering before a diagnosis (and therefore treatment) may be considered. For example, it has been stated that Orthostatic Hypotension (OH) is the most debilitating symptom of Autonomic Neuropathy . The clinical definition of OH is a 20/10 mmHg decrease in BP upon assuming an upright posture (i.e., sitting or standing up). A normal change in BP upon standing is a 10% increase in BP (e.g., approximately an 11/7 mmHg increase , assuming a normal average resting BP). Therefore a weak increase is already abnormal, and a lack of change in BP upon standing is already significantly abnormal. Yet we are forced to make our patients wait (and suffer) until the abnormal BP change becomes a threefold a
a Normal is a 10 mmHg increase in systolic BP, and the criterion for OH is a 20 mmHg decrease in systolic BP. The net is a 30 mmHg decrease. Comparing the abnormal decrease (30) to the normal increase (10), the result is a threefold abnormal change, and that is just the threshold for a clinical diagnosis.
or greater abnormality. bb Waiting for the clinical OH criteria may accelerate the onset of Heart Failure. The decrease in BP upon standing may also cause poor cardiac perfusion and reduced Diastolic pressure. The resulting poor brain perfusion often leads to Sympathetic stimulation that increases Systolic pressure. If left undetected, the Systolic-Diastolic difference (Pulse Pressure) may eventually grow to Hypertension and Heart Failure (two common comorbidities associated with Diabetes). In these cases, when the Hypertension and Heart Failure are treated with Sympatholytics, the Sympatholytics may further exacerbate the condition.
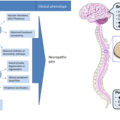
More information is needed.Autonomic neuropathy references the progression from healthy to end stage. The end stage is known as Cardiovascular Autonomic Neuropathy (CAN, see Fig. 10.1 ). This terminology is unfortunate in that “neuropathy” is often misleading. The perception of “neuropathy” is “dead nerves.” The teaching is that dead nerves are not treatable. Therefore the logic is that Autonomic Neuropathy is not treatable. This is compounded by the fact that the function of the two individual Autonomic branches, the P&S nervous systems, has been difficult to measure independently and simultaneously, without assumption and approximation. The result is that Autonomic Neuropathy is largely unknown. However, recently, patients have been seeking solutions to long-standing conditions and have driven an increased awareness.
Regardless of the terminology, and contrary to the common misperception, Autonomic Neuropathy is treatable, even in the end stage ; P&S balance is the key. To help with the misperception, where appropriate, we will use “Autonomic Dysfunction” or more specifically “P&S Dysfunction” as the “new name” for “Autonomic Neuropathy.” Hopefully, this will also help with earlier detection. The earlier P&S Dysfunction is detected, the greater the number of available therapy options, including supplements and lifestyle modifications as well as pharmaceutical therapies. While it is easier to correct early-stage P&S Dysfunction compared with advanced-stage autonomic neuropathic damage, now that we are able to measure P&S Dysfunction in all stages, all stages are treatable .
Autonomic function
The main function of the Autonomic Nervous System (ANS) is to maintain homeostasis, regardless of the conditions. The P&S dynamically adjust their output to maintain homeostasis and apparent normalcy even in the face of degraded P&S function or degraded end-organ function. The P&S nervous systems control and coordinate organ function. Organ dysfunction generates symptoms (typically not P&S dysfunction). The P&S nervous systems control and coordinate normal organ function even when they are in abnormal states. c
c For example, assume proper organ control requires a P&S activity of 4. Given normal conditions, with proper P&S balance, that would be a P-activity of 2 and an S-activity of 2 (2+2=4). All is normal and there are no symptoms. However, if S-activity becomes excessive (i.e., SE) and rises to a 3, and P-activity degrades to a 1, normal organ function is still maintained because P&S activity is still a 4 (3+1=4), and there are yet to be any symptoms. An ultimate possibility is when SE rises to a 4, and P-activity degrades to a 0. Normal organ function is still maintained because P&S activity is still a 4 (4+0=4), and there are still no symptoms until the ultimate symptom, Sudden Cardiac Death, because there is no longer sufficient P-activity to prevent a Sympathetically mediated Ventricular Tachyrhythm from progressing. Since we know that Diabetes leads to P&S Dysfunction, then we should monitor P&S activity more frequently and right from the beginning, even when asymptomatic, to detect the earliest P&S dysfunction and treat to reestablish and maintain P&S balance.
(Note, if the two P&S branches are measured independently and simultaneously, it would enable the ability to detect dysfunction earlier.) The Sympathetics are the reactionary branch and react to the threshold set by the Parasympathetics. With P&S Dysfunction, due to Diabetes for example, these P&S adjustments result in P&S imbalance and begin to affect other systems within the body, including the cardiovascular, renal, and immune systems, and compound the effects of the disease itself; Diabetes in this case. This is, in part, the basis for the constellation of symptoms known to degrade Quality of Life (QoL) in patients diagnosed with Diabetes (as well as many other chronic diseases). By the time symptoms present as a result of end-organ dysfunction or failure, the P&S have been abnormally out of balance for considerably longer, but are still treatable.By way of an example, consider the thermostat on the wall of your house. Assume that you have no knowledge of, and cannot access or hear, the separate heating and cooling systems of your house. Further, assume that you like your house temperature to be 70°F (like heart rate at 70 bpm), and that is what your thermostat is set to. As long as the thermostat reads “70,” everyone is happy. Now assume it is a hot, humid, August day in a southern US city, the cooling system is working well, but a $5 heater switch fails, and the heater comes on full. What happens to the temperature in the house as read on the thermostat? … Nothing! … The cooling system is working well, so it “amps-up” and compensates, not only for the ambient temperature, but also for the dysfunctional heating system, and 70 is still the reading. If temperature was heart rate, the heart rate does not change even though both autonomic branches are overactive. This is more information and potentially earlier detection.
The question becomes, how long will the dysfunctional situation last until there is a catastrophic failure (costing $5000 or more) in one or both of the cooling and heating systems? Meanwhile, you are totally unaware (like your heart rate is still normal, 70) until the catastrophic failure (like a heart attack), when it is too late. Monitoring the P&S nervous system independently and simultaneously will detect when the $5 switch fails. Unfortunately, most noninvasive, autonomic measures only measure total autonomic function. They only measure the resulting temperature as read on the thermostat, and the underlying abnormality is missed until it is late in the progression. From monitoring the individual branches, therapy recommendations (as described herein) help keep the systems functioning normally (healthy) and a proper balance between the cooling (Parasympathetic) and heating (Sympathetic) systems maintained, to help keep the “$5 switches” functioning normally, helping to maintain both health and wellness. The technique known as P&S Monitoring monitors the switches (and more) as well as the thermostat.
Autonomic dysfunction and morbidity and mortality risk
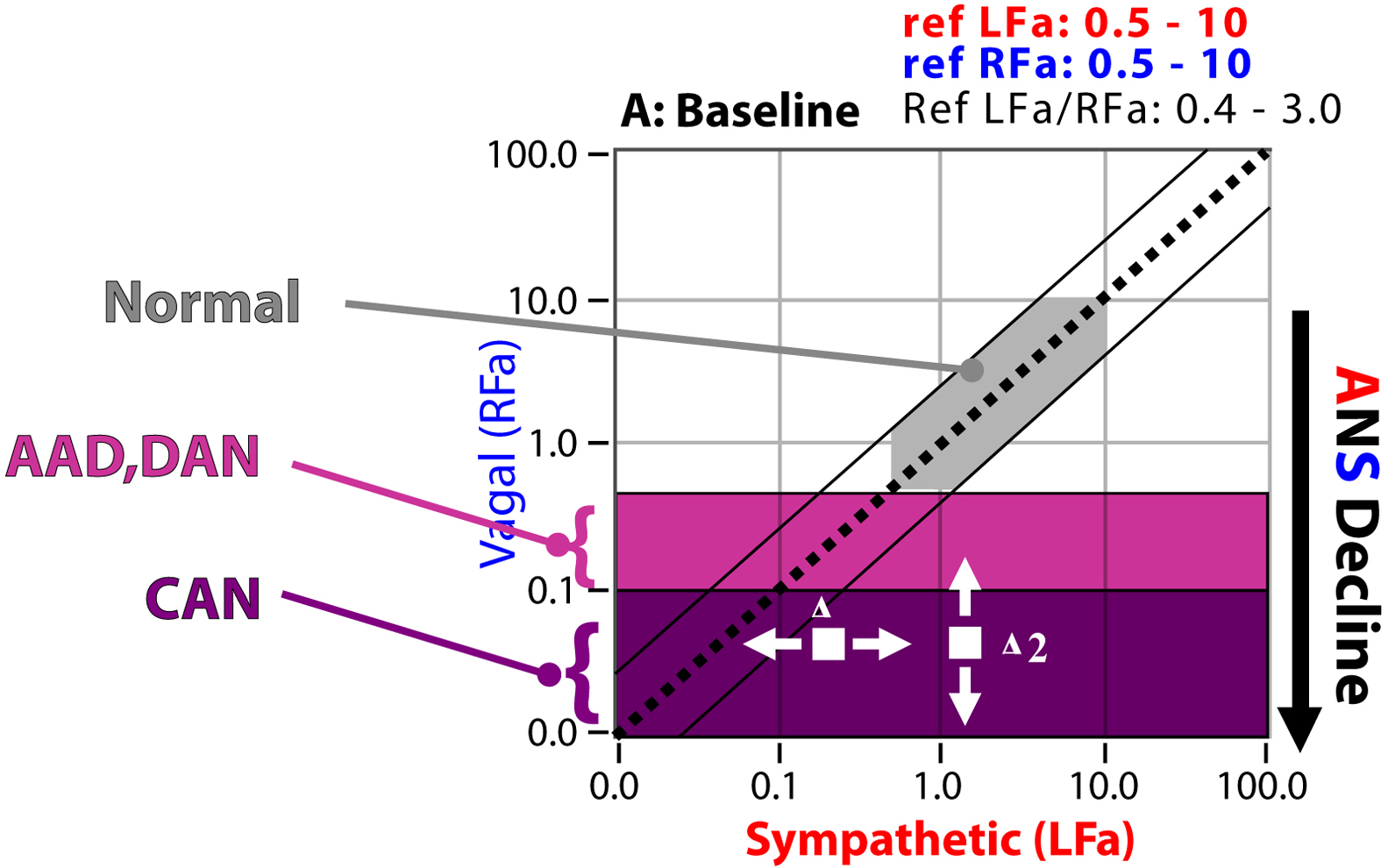
Again, CAN is end-stage P&S Dysfunction (see Fig. 10.1 ). CAN leads to the catastrophic failure in the above model. It is approximated by a severe lack of HRV, which is by definition a severe lack of resting Parasympathetic activity. d
d CAN is defined as the Parasympathetic measure (RFa, or Respiratory Frequency area) < 0.1 bpm 2 . This indicates that there is insufficient Parasympathetic activity to prevent, for example, a Sympathetically mediated Ventricular Tachyrhythm from progressing. In other words, the mortality risk is high as in the Framingham Heart criteria.
CAN places patients at risk for increased morbidity and mortality. The large cohort Framingham Heart Study provided evidence in the general population that lack of HRV is associated with increased mortality risk. Decreased HRV is associated with a number of clinical conditions, including Diabetes and many of its comorbidities, such as Coronary Artery Disease (CAD) , Hypertension , altered Ventricular function , and premature aging . Mortality after a myocardial infarction (MI) is more strongly associated with decreased Parasympathetic tone (decreased HRV) than with poor systolic function . Decreased Parasympathetic tone is an independent predictor of mortality in Congestive Heart Failure . Cardiovascular diseases are highlighted here since Diabetes typically involves earlier cardiovascular disease as an indication of increased mortality risk.With P&S Monitoring, early-stage Dysfunction (the $5 switch failure in the thermostat model) may now be detected early and clinically followed, before the onset of CAN (the $5000 failure in the thermostat model). When P&S balance is restored, by normalizing the ratio of resting S-activity to resting P-activity (known as Sympathovagal Balance, or SB=S/P), the progression of P&S Dysfunction is slowed or stayed. As you know, aging is dictated by an individual’s history, lifestyle, and genetic make-up. The normal aging process (without the acceleration due to chronic disease or disorder) causes autonomic decline, ultimately leading to CAN. This normal aging process is the target rate of autonomic decline. This is one reason why even “healthy” older people have greater morbidity and mortality risk than younger people. CAN is inevitable for all and each individual is different.
Even after the onset of CAN, normalizing SB (see Fig. 10.1 ) slows the progression of P&S Dysfunction by limiting the morbidity and mortality risk, optimizing QoL, and promoting normal longevity for the individual. Establishing and maintaining normal SB, appropriate for the individual, also reduces secondary symptoms, which reduces medication load, prevents hospitalizations and re-hospitalizations, and thereby lowers healthcare costs .
CAN is associated with high risk of sudden cardiac death and other Major Adverse Cardiovascular End-points (MACE). In patients with Diabetes, Diabetic Autonomic Neuropathy (DAN) indicates an increased risk of CAN and is diagnosed based on the early symptoms of neuropathy and P&S Dysfunction (see Fig. 10.1 ). DAN involves lightheadedness (or dizziness, arguably the worst of the symptoms), as well as nervous system dysfunction, vascular dysfunction, gastrointestinal (GI) upset, BP dysfunction, vision decline (faster than presbyopia), Anhydrosis, dry and cold skin, sex dysfunction, renal dysfunction, mental and cognitive dysfunction, and cardiovascular disease . Much of the sensory and motor dysfunctions (including wound healing difficulties and amputation issues) are a function of poor autonomic nerve control of the vasculature. This causes vascular dysfunction, leading to poor tissue perfusion, predisposing the patient to acquiring wounds and not having the sensation of the wound’s existence (sensory nerves need blood also), facilitating infection (due to the poor perfusion and again without sensation) and often ending in amputation. Clinical symptoms generally do not appear until long after the onset of Diabetes. However, subclinical P&S Dysfunction may occur within a year of diagnosis in type 2 Diabetes and within two years in type 1 Diabetes . In general, a diagnosis of DAN is made only after eliminating other causes of neuropathy.
CAN is defined as very low, resting Parasympathetic activity (see Fig. 10.1 ). CAN indicates that the Parasympathetics maybe too weak to prevent a Sympathetically mediated Ventricular Tachyrhythm from progressing. Thus CAN with high, resting Sympathetic activity relative to Parasympathetic activity is a mortality as well as a morbidity risk indicator (e.g., silent MI, respiratory failure, and sudden death) . The mortality risk for DAN has been estimated to be 44% within 2.5 years of diagnosing symptomatic autonomic neuropathy . Meta -analysis data for the pooled prevalence rate risk for silent myocardial ischemia was 1.96, with 95% confidence interval of 1.53–2.51 ( P <.001; n =1468 total subjects). Thus a consistent association between autonomic neuropathy and the presence of silent myocardial ischemia was shown .
There are two forms of CAN: functional and structural (see Fig. 10.1 ). Functional CAN patients often find (temporary) relief of CAN once a proper P&S balance is restored for them, the individual patient. Structural CAN is not reversible. Both carry mortality risks. CAN risk is the same as for post-MI and post-coronary artery bypass graft (CABG) patients, as documented in the Framingham Heart Study , as well as for other MACE (including stroke) patients. CAN has been found to carry a 50% increase in the 2-year mortality rate .
CAN is typically normal for post-MI, post-CABG, chronically ill, and geriatric patients. CAN is a normal part of the aging process on the ANS. Again, CAN simply indicates mortality risk. In other words, it is a sign of age, and people with CAN, who tend to be older, have a greater risk of dying than people without CAN, who tend to be younger and healthier. However, regardless of age, CAN with abnormal P&S balance (SB) is not normal. CAN with high SB is high risk and is associated with other risk factors , including (1) low ejection fraction ; (2) poor cardiac output ; (3) arrhythmias ; (4) cardiomyopathies , including chronic heart failure ; (5) poor circulation , including poor cardiac circulation (Angina or CAD) ; (6) greater mortality ; and (7) greater morbidity , including silent MI and early cardiac death . CAN with low P&S balance is associated with “Broken Heart Syndrome” , depression, and depression-anxiety syndromes. Often, CAN leads to the need for cardiac intervention or an implanted cardiac device. CAN affects longevity.
A history of assumption and approximation quantified
Unfortunately, early signs of P&S Dysfunction are often not recognized due to two main reasons. First, the current understanding of the effects of the ANS and its interaction with other physiological systems is incomplete and therefore few therapies are designed for the ANS. Second, a reliable clinical tool to measure and monitor the P&S nervous systems, independently and simultaneously, without assumptions or approximations, did not exist until recently . Prior to this, Autonomic Neuropathy could be clinically diagnosed [OH, Gastroparesis, Shy–Drager, Postural Orthostatic Tachycardia Syndrome (POTS), etc.] only at an advanced stage with dramatic symptoms. At this stage, it is usually much too late for anything but treatment of the symptoms. With simultaneous, independent measures of P&S branches, known as P&S Monitoring (Physio PS, Inc., Atlanta, GA), these patients can be identified even when they are still asymptomatic, or mildly symptomatic, a fairly common situation usually accompanied by lightheadedness, fatigue, palpitations, difficult-to-manage hypertension, memory and cognitive difficulties, sleep difficulties, and so on. Therapeutic intervention seems to improve outcomes by balancing P&S activity for the individual and slowing or halting autonomic decline and the associated disease progression.
The history of noninvasive ANS monitoring in clinical practice is confusing. Traditionally, it has been based only on measures of cardiac beat-to-beat analyses (HRV, or beat-to-beat BP). Measures of HRV, as defined in the 1996 Circulation standards article , are mixed or incomplete measures of the P&S. This is also true for beat-to-beat BP. This is not surprising, as HRV or beat-to-beat BP by themselves, regardless of how many components are computed, are but one independent measure of a system (the ANS) that contains two components: P&S. From a mathematical perspective, one measure is insufficient to fully characterize a two-component system. If one measure changes, it is impossible to determine which component changed without making assumptions and approximations or without additional information. This has resulted in a very low clinical acceptance rate for this method, except in extreme cases. The common assumption is that the Parasympathetics are very, very low as compared with the Sympathetics, but this may not be true after therapy, therefore follow-up testing is not revealing or worse, misleading. HRV or beat-to-beat BP alone provides no additional information. The use of HRV or beat-to-beat BP alone merely indicates the obvious: that the patient’s ANS is or is not functioning well.
Another consequence of a lack of proper measurements is that there is little data on the P&S individually, so there are very few medications that are approved for the P&S. In fact, only two medications are approved specifically for Dysautonomia [Midodrine (ProAmatine) and Northera (Droxidopa)] and their approval is limited to only Neurogenic Orthostatic Hypotension (NOH). All other therapies for P&S Dysfunction are off-label, supplements, or lifestyle modifications . P&S Dysfunction may now be diagnosed earlier and followed serially and treated, including titrated based on the individual patient’s measured responses . It is hoped that newer, improved protocols may be developed.
There is yet another poorly understood issue. The P&S are dynamic systems. Even when an individual is at rest, the P&S is not resting. While the Parasympathetic nervous system is considered the “Rest and Digest” nervous system, it is not resting while we are resting. In fact, it may be argued that the P&S is more active when we are sleeping than while we are awake and active. This is an issue because, for the vast majority of tests and times of assessment, patients are at rest (seated or supine). The average patient with Dysautonomia has seen over a dozen doctors over years to decades with little to no relief. To help restore some faith in the healthcare system, it is explained to them that they have been assessed largely at rest, and they are shown their P&S assessment results that demonstrate that the patient is (typically) “normal” at rest. “They better be, they have had many doctors over many years working very hard to ensure they are normal … at rest.” Their problems typically arise from abnormal P&S responses while active.
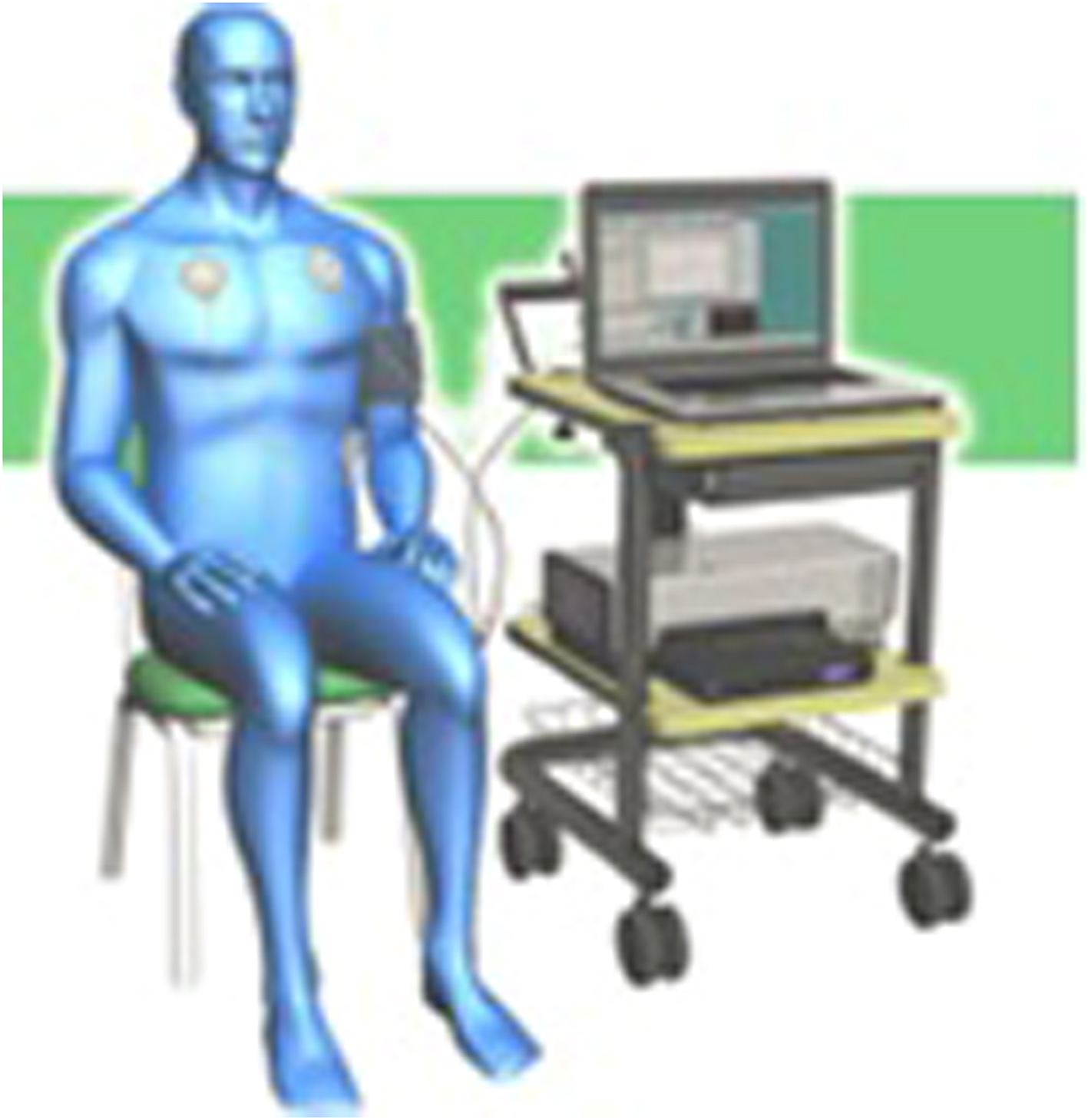
The basis for the P&S assessment test is four challenges separated by resting baselines. First, there is the resting (baseline) challenge where the patient is relaxed and breathing normally, (typically e
e Patients who cannot stand up are to lay supine during the seated phases of the test and then asked to sit with proper posture during the stand phase of the test.
) sitting back and relaxed in a stationary, well supported chair. This is equivalent to supine and establishes the patient as their own control so that they may be treated starting the same day. It also establishes the classical autonomic measures for Autonomic Neuropathy (DAN and CAN) and measures SB (see Fig. 10.1 ). While still at rest, sitting or supine, the Parasympathetics are independently challenged with a minute of paced breathing (deep breathing) at 0.1 Hz or six-breathes per minute. After a brief rest period of normal breathing, to allow the P&S to return to baseline, the Sympathetics are independently challenged with a series of five short Valsalva maneuvers. As you may remember, Valsalva maneuvers are taught as strong Parasympathetic challenges, and the long ones (>20 seconds) are indeed. Short Valsalva maneuvers (<20 seconds) are strong Sympathetic challenges. Of course, there is a tacit challenge to the opposing autonomic branch inherent in these two challenges as well, which may also highlight Dysautonomia. After another brief rest period of normal breathing, to allow the P&S to return to baseline, both P&S branches are challenged together in a postural change challenge: standing up or tilt-test, or if wheel chair bound, sitting-up from a supine position. ff Patients that may need a cane or crutch or some other device to assist with stand are welcome to use it. If patients feel lightheaded or dizzy while standing and do not feel they are able to continue, they are welcome to sit back down to avoid fainting and finish the test seated. This may not only capture reasons for the lightheadedness, but also the recovery from lightheadedness.
This not only helps to document any cause of lightheadedness (Orthostatic Dysfunction or Syncope), it also helps to document how well the two ANS branches coordinate. If they do not coordinate for something as common as standing up, they are not likely to coordinate well when it comes to controlling the organs or organ systems. With this simple, safe, and comfortable office-based clinical study, reliable and repeatable P&S data are available both from the resting patient and the challenged patient for a greater diagnostic yield.
Diabetes: also considered a model of the effect of chronic disease on P&S activity over time
Natural history of autonomic decline
Normal autonomic decline
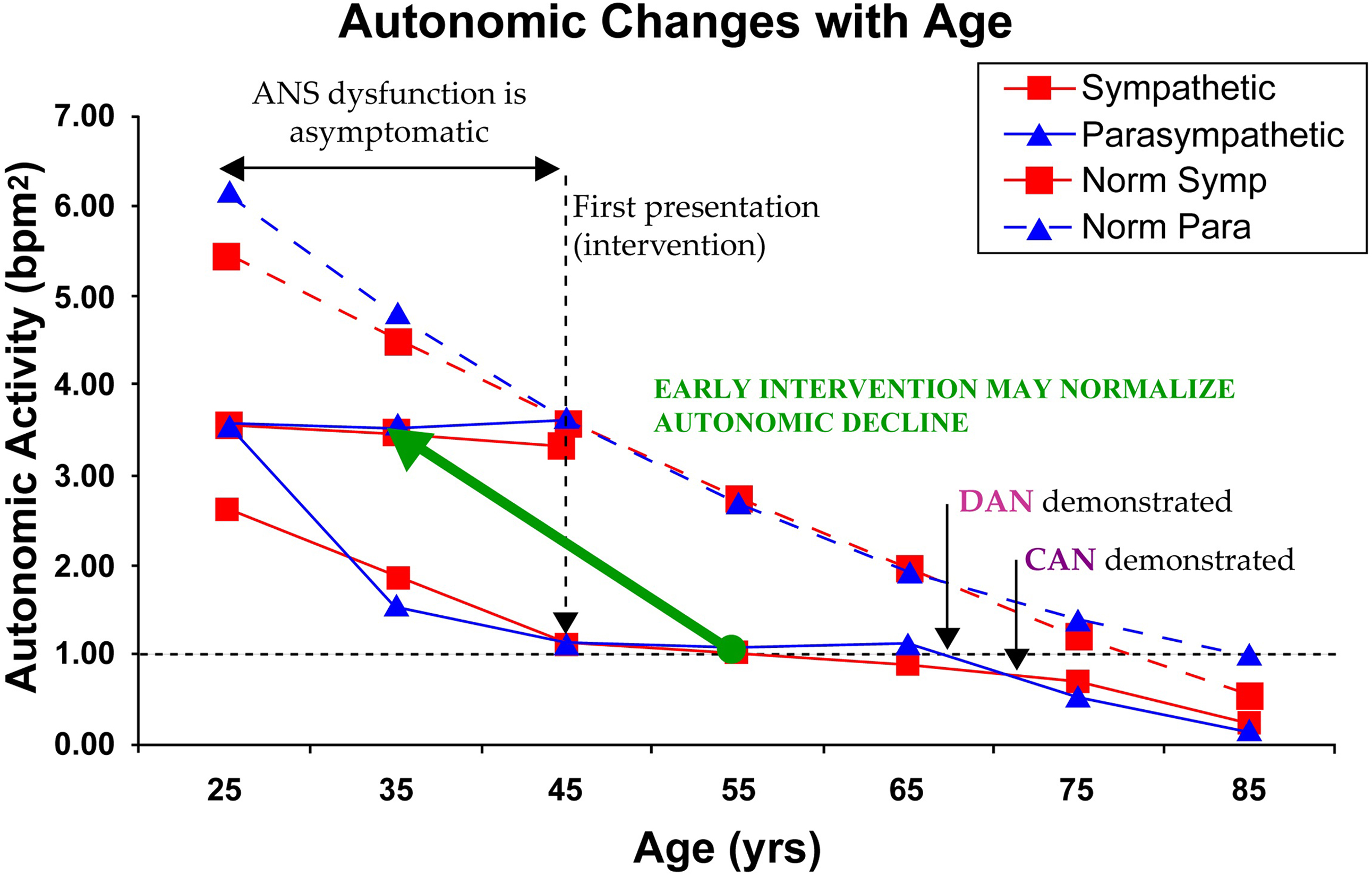
As mentioned above, autonomic decline is a fact of life (see Fig. 10.2 ). Humans are born with as active a P&S nervous system as they will have. Ultimately, at the end of life, there is no activity in the P or S nervous systems. The normal aging process causes a gradual decline over time. Fig. 10.2 displays the normal Parasympathetic (broken, blue curve) and normal Sympathetic (broken, red curve) changes over time from 346 known healthy volunteers from ages 3 to 96years (58% female). None of these subjects are diagnosed with chronic disease and all demonstrate normal responses to the clinical P&S assessment study. P&S activity is presented on the ordinate (in bpm 2 ) and age (in years) is presented on the abscissa. The broken, black line at the 1.0 level is the normalized threshold for DAN or Advanced Autonomic Dysfunction (AAD, if not diabetic), based on Framingham Heart Study findings. These curves offer several insights to normal autonomic decline. At both ends of the age range, Parasympathetic activity is normally higher than Sympathetic activity (SB g
g SB=(resting Sympathetic activity)/(resting Parasympathetic activity), Normal range: 0.4<SB<3.0; Preferred normal for geriatric and chronically ill: 0.4<SB<1.0; Preferred normal for younger patients: 1.0<SB<3.0.
is in the low-normal range: 0.4<SB<1.0). In the early years, this reflects the Parasympathetic involvement in development. In the later years, the low-normal SB reflects the added Parasympathetic activity that has been shown to protect the organs, reduce morbidity and mortality, and improve outcomes . This has become the recommended P&S balance for geriatric patients. In the middle years, the balance is approximately 1.0 (the curves overlie each other) or a little above 1.0. This may reflect the need of young adults to have the additional energy for child-rearing.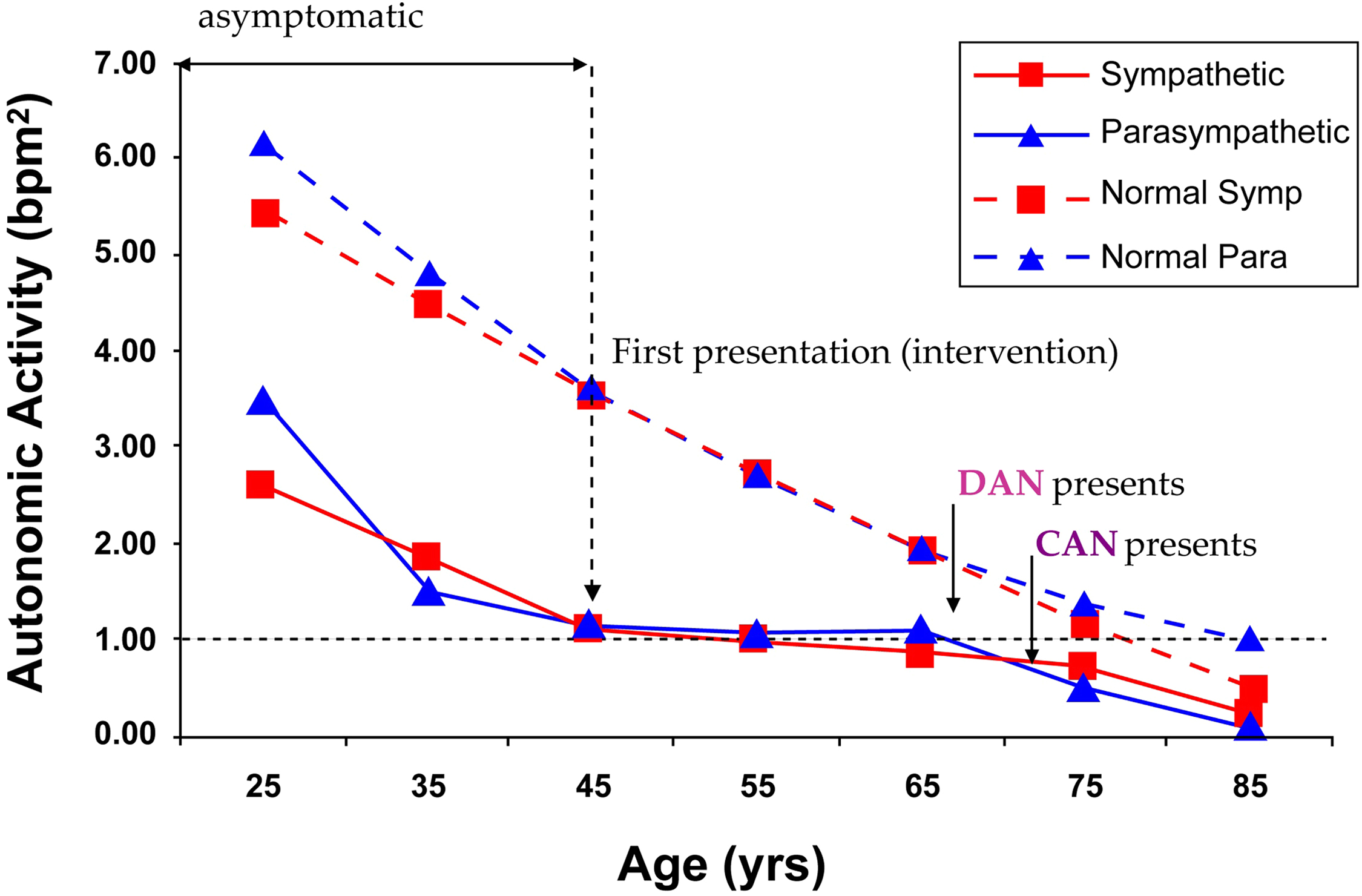
Diabetes effect on autonomic decline
The effect of Diabetes Mellitus on the ANS may be the most studied effect of all chronic diseases. Fig. 10.2 also displays the Parasympathetic (solid, blue curve) and Sympathetic (solid, red curve) changes over time from 1483 patients diagnosed with type 2 Diabetes from ages 23 to 86 years (51% Female). Diabetes’ effect on P&S function is to accelerate P&S decline (see Fig. 10.2 , compare normal and patient curves), possibly causing earlier morbidity and mortality risk. The key points from the patient curves in Fig. 10.2 are:
- •
On average, P&S levels are approximately 50% depleted upon first diagnosis, and it is asymptomatic .
- •
This seems to reflect the American Diabetes Association’s (ADA’s) indication that by the time of diagnosis, a type 2 Diabetes patient has had the disease for up to five years .
- •
This confirms the ADA recommendation for P&S Monitoring within two years of a diagnosis of type 2 Diabetes .
- •
The seven years (five plus two) of the disease and the prediabetic conditions have already significantly depleted the P&S, before the disease is even detected.
- •
On-going research is looking for earlier indications (e.g., Prediabetes and Metabolic Syndrome) to indicate even earlier monitoring and intervention.
- •
- •
- •
On average, by time symptoms present, P&S levels are approximately 80% depleted, and the decline was asymptomatic .
- •
Diabetes seems to cause late-stage (relative) Sympathetic dominance (SB > 2.5). The Sympathetic (red) curve is higher than the Parasympathetic (blue) curve for the patients after approximately age 72, whereas, for the normal subjects, the Parasympathetic (blue) curve is higher than the Sympathetic (red) curve after approximately age 68. Again, high SB with CAN is known to increase morbidity and mortality, including Sudden Cardiac Death . The age-matched, older, normal subjects demonstrate low-normal SB (0.4<SB<1.0). Low-normal SB is known to reduce morbidity and mortality . Low-normal SB may be titrated . The choice of therapy is dependent on the patient’s history.
- •
Intervention slows P&S decline.
- •
Therapy to establish and restore normal SB, especially early, is known to slow autonomic decline. Note how flat the patients’ curves ( Fig. 10.2 ) are after “first presentation” and intervention around age 45, until decline continues (in parallel with normals) around age 65, when aging apparently catches up.
- •
If therapy is adopted earlier ( Fig. 10.3 ), it seems as if therapy could translate the patient’s P&S response curves to the more normal curves of the healthy subjects, minimizing morbidity and mortality, and improving outcomes.
- •