17. Diabetes
Lindsay Oliver and Nick Lewis-Barned
LEARNING OBJECTIVES
By the end of this chapter the reader will be able to:
• Understand the nature, treatment and dietetic interventions appropriate in the management of diabetes;
• Be clear about the different types of diabetes and their unique management;
• Be aware of the long-term health implication and cost of diabetes and how risk factors can be managed;
• Be aware of the historical management of diabetes, and also of future developments; and
• Apply theoretical information to a case study.
Introduction
Food and lifestyle management is core to the prevention of type 2 diabetes and to the treatment of risk factors associated with the progression and development of long-term complications in all types of diabetes. However, agreement about what dietetic interventions are best is still debated, particularly in type 2 diabetes. Pragmatically it may be better to focus on the patient’s own therapeutic goals and consider which dietetic intervention is evidence based in that instance.
Treatment goals could include any or all of the following:
• Blood glucose levels in the normal range, or as close to normal as safely possible (4.5–8 mmol/L pre-prandial) with an ideal HbA1c of 6–7% (DCCT) or 42–59 mmol/mol (IFCC);
• A lipid profile that reduces vascular disease (total cholesterol < 4 mmol/L, low density lipoprotein (LDL) < 2 mmol/L, triglyceride (TG) < 2 mmol/L);
• Blood pressure control (130/80 mmHg or 125/75 mmHg for those with renal impairment/microalbuminuria/proteinuria);
• Weight management (prevention of weight gain or weight loss);
• Maintenance of nutritional status particularly where other diagnosis are present; and
• Sustainability of dietary modifications by planning realistic, patient-centred changes.
When considering the options for individuals with diabetes, their current behaviour, barriers and goals need to be thought through, to develop concrete, individualised action plans.
In this section we will attempt to outline some of the differences in dietetic messages in relation to individual goals of treatment and the underpinning physiological problem.
Also refer to Chapter 18 on Obesity and Chapter 19 on Cardiovascular Disease.
Historical context
The first writings on diabetes were about diet and the earliest observers note that it was associated with dietary excess and advocated lifestyle change. Diet and lifestyle were the only options for managing diabetes until the discovery of insulin, in 1920, heralded the era of life-saving treatment for type 1 diabetes. From a dietary perspective this led to a focus mainly on carbohydrate. For almost five decades the importance of vascular risk management through food choice, weight management and physical activity, especially for those with type 2 diabetes, was largely overlooked. As technological advances in self glucose monitoring and insulin delivery have occurred, nutritional options have also developed.
Our method of measuring HbA1c is presently undergoing change. The Diabetes Control and Complications Trial (DCCT) values expressed as a % are being replaced with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) values expressed as mmol/mol. At present both values are reported (www.diabetes.nhs.uk).
Pathophysiology and medical management of diabetes
Type 1 and type 2 diabetes are characterised by high levels of circulating glucose. Both arise from physiological problems with insulin whose main role is to transport glucose from the blood into cells either for use in metabolic processes or for storage as glycogen. However, in other respects the underlying causes of type 1 and type 2 diabetes are fundamentally different and this gives rise to different presentation and management (Table 17.1).
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| Pathophysiology | Autoimmune destruction of β-cells in pancreatic islet tissue results in loss of insulin secretory capacity and absolute insulin deficiency | Insulin insensitivity (insulin resistance) gives rise to compensatory high insulin production. Secondary inability of insulin secretion to be maintained results in progressive hyperglycaemia due to relative insulin deficiency |
| Prevalence1 | The prevalence for type 1 diabetes is estimated as 0.5% in the UK, with an annual incidence in of 14/100,000 in children. Risk is increased approximately 10-fold in first-degree relatives, but unexplained geographical variation and migrant studies suggests that environmental factors are also very important, but not well understood | The prevalence is 4–5% of the UK population. Risk factors include a family history of type 2 diabetes, age, increasing weight, physical inactivity. Type 2 diabetes is more common in ethnic minority groups in the UK. Between 25 and 30% of those with type 2 diabetes are undiagnosed |
| Typical features at presentation | Although β-cells failure may take place gradually over many years, onset is often abrupt with typical symptoms of hyperglycaemia occurring over a few weeks. About 10% present with severe insulin lack leading to marked hyperglycaemia, and fat breakdown. This results in formation of acidic ketone bodies (ketoacidosis), which is a medical emergency requiring intravenous fluid, insulin and electrolyte replacement | Glucose levels increase gradually over many years. It is possible to identify a ‘latent’ period of glucose abnormality before diabetes develops. Onset is therefore insidious and often asymptomatic. While typical symptoms of hyperglycaemia may occur, type 2 diabetes is often diagnosed as a result of active screening of high-risk groups |
| Management | Insulin replacement is always required. This must be given by subcutaneous injection. Peaks and troughs in the circulating insulin levels need to match the effects of timing and amounts of carbohydrate, and this forms the basis of dietary management | Initially this is directed at reducing insulin resistance through increased physical activity and weight management. Most patients will in time require tablet therapy either to reduce insulin resistance (metformin and thiazolidinediones) or to enhance insulin production (suplhonylureas). Thirty to 50% of patients will require supplementary insulin. Dietary management of cardiovascular risk and insulin resistance, however, remain key elements of management |
Despite this, type 1 and type 2 diabetes can sometimes be difficult to distinguish at presentation due to overlap in age, weight and symptoms. Initial management may be based on a clinical assessment of the likely type of diabetes, and careful observation of response to treatments. Where the type of diabetes is unclear, antibody tests for type 1 diabetes can be helpful.
Other forms of diabetes
While type 1 and type 2 diabetes account for the majority of those with diabetes, this can also arise from other causes.
Gestational diabetes
Gestational diabetes is defined as diabetes or impaired glucose tolerance that is identified for the first time during pregnancy and resolves following delivery. This is due to physiologically increased insulin resistance in pregnancy. Hyperglycaemia not only influences growth and development of the fetus, but also identifies affected women who are at increased risk of future type 2 diabetes. Management is dietary, although some women also require insulin treatment.
Pancreatic failure
Direct damage to the pancreas due to surgery or pancreatic inflammation (pancreatitis) may result in both insulin deficiency (partial or complete), and pancreatic enzyme deficiency causing malabsorption. This complex situation requires careful management of both aspects of pancreatic failure.
Monogenic diabetes
Formerly known as maturity onset diabetes in the young (MODY), this describes a rare group of inherited types of diabetes. All are characterised by non-insulin requiring diabetes with onset in childhood or young adulthood, and an autosomal dominant pattern of inheritance.
Long-term complications of diabetes
Glucose-related complications (microvascular complications)
High glucose levels over 4 or more years increase the risk of diabetes specific complications. 2,3 These result from structural and functional changes, including glycosylation of proteins and occlusion of small blood vessels. This most commonly affects light-sensitive retinal cells in the eye, renal glomeruli and peripheral nerves, resulting in visual impairment, kidney damage and pain or sensory loss especially in the feet. The degree of risk is directly related to the degree of glucose abnormality, with near normal glucose control minimising these risks. Systematic screening for microvascular complications and early treatment are part of routine care.
Vascular risk (macrovascular complications)
Both types of diabetes, but especially type 2 diabetes, are associated with a magnified risk of atherosclerosis resulting in heart disease, stroke and peripheral vascular disease. 4 While glucose control plays a part in this, the major contributors to risk are the same as for people without diabetes (blood pressure, lipid abnormalities, family history and age) except that the effects are magnified, and the relative protection experienced by premenopausal women is lost. Overall people with diabetes are at a two- to fourfold increased risk of macrovascular disease. Active management of these risk factors plays a key role in preventing macrovascualar complications. Food choices, lifestyle and drug management are aimed at optimal control of risk factors.
Cost of diabetes care
Current estimates suggest that direct health cost of diabetes accounts for about 5% of the UK health budget, not including social costs related to sickness and disability and more than 10% of hospital bed days. These are largely the result of complications and their management. Government policy has increasingly focused on early identification, systematic care and self-management to reduce the personal and financial burden of this. 5
Nutritional therapy in the management of diabetes
Prevention of diabetes
Preventative approaches to type 1 diabetes have so far all proved unsuccessful. Although environmental factors are thought to be important, type 1 diabetes is not directly caused by individual lifestyles and so there are no preventative lifestyle measures or strategies that can be implemented. 6
However, the current epidemic of type 2 diabetes is likely to reflect lifestyle choices and increasing rates of overweight and obesity, with genetics playing a smaller but important role.
Some groups of the population have a higher risk of developing diabetes (see Table 17.2). Individuals in these categories should be screened for diabetes on a regular basis, although there is no evidence base to suggest how often. In practice it is suggested that fasting blood glucose should be checked annually. The risk of developing diabetes also increases with ages and is higher in some ethnic groups (Box 17.1).
| Risk factor | Evidence-based strategies |
|---|---|
| Weight management10 | 600-cal deficit approach Increased physical activity Reduced fat and alcohol Portion size Anti-obesity drugs Bariatric surgery |
| Lipid profile11 | Reduced saturated fat (and trans fatty acids) Moderate monounsaturated fat Weight management |
| Cardio-protective messages | Eat fish containing omega-3 fatty acids (1–2 portions weekly, depending on presence of CVD) |
| Blood pressure12,13 | Weight management Moderate alcohol Reduce salt DASH style pattern of eating (low saturated fat/7 portions of fruit and vegetables daily) |
 Box 17.1
Box 17.1 Positive family history of type 2 diabetes
Personal history of:
• Gestational diabetes
• Impaired glucose tolerance
• Impaired fasting glucose
• Vascular disease (MI, PVD, angina, CVA, TIA)
• Central obesity (waist circumference: male > 100 cm, female > 90 cm)
• Increased blood pressure (pretreatment of > 130/85 mmHg)
• Increased lipid profile (HDL male < 1.0 mmol/L, female < 1.2 mmol/L fasting triglyceride> 2.0)
Iatrogenic factors: pancreatic surgery or steroid treatment
People with impaired glucose tolerance (pre-diabetes) not only have a 10% annual risk of developing diabetes, but also have increased cardiovascular risk. This can be calculated using Joint British Societies risk tables. 7
It is clear that lifestyle modification can prevent or delay type 2 diabetes. Both the Finnish Diabetes Prevention Study and the Diabetes Prevention Program in the USA strongly support this. 8,9 Lifestyle modification has been shown to be twice as effective as metformin in the prevention of type 2 diabetes and the effects of lifestyle change were also shown to be additive, i.e. the more changes made, the more risk reduction.
Key recommendations from these programmes included:
• Moderate weight loss (5–7% of body weight);
• Regular physical activity (150 min/week); and
• Reduced fat intake (primary method of calorie reduction).
Of note, reduction of saturated fat has a strong, energy-independent impact on insulin resistance and so reduction of saturated fat should be the main focus of fat reduction. Whole grain foods have also been associated with improved insulin sensitivity, but studies looking at glycaemic index/load have been inconclusive in terms of preventing diabetes. 6
Impaired glucose tolerance (IGT) and type 2 diabetes
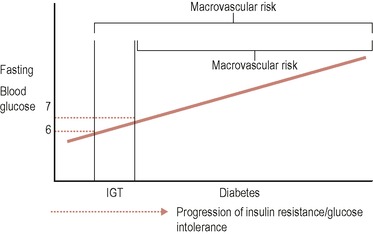
IGT is not a disease in itself, but it is an indicator of risk for both cardiovascular disease (CVD) and type 2 diabetes. Individuals with IGT usually have features of the metabolic syndrome, including insulin resistance, and which is strongly associated with risk of developing diabetes and CVD (macrovascular risk). Once individuals progress to develop frank diabetes, they have blood glucose levels that, if not managed, can additionally lead to microvascular disease. (See Figure 17.1.)
 |
| Figure 17.1 • |
Management of impaired glucose tolerance
The goal of managing IGT is twofold and is aimed at:
1. Reducing the risk of progression to diabetes by reducing insulin resistance:
Insulin resistance has been shown to be made worse by increased weight, waist circumference and saturated fat intake. Physical activity improves insulin resistance.
2. Managing cardiovascular risk factors:
People with IGT should have their cardiovascular risk assessed using Joint British Society tables. Smoking cessation, aspirin, blood pressure management and the use of statins and cardio-protective dietary measures are all important aspects of risk reduction. They should be tailored to the risk profile of the individual. Weight management will have a global impact on all risk factors other than smoking.
There is a lack of dietary evidence specific to diabetes, IGT and cardiovascular risk, and so evidence is primarily extrapolated from more general studies (Table 17.2).
(See related chapter on cardiovascular disease.)
Management of type 2 diabetes
Individuals with type 2 diabetes usually have many of the features of metabolic syndrome and so will need monitoring, assessment and treatment of cardiovascular risk factors. In addition, they are now at risk of microvascular disease due to hyperglycaemia.
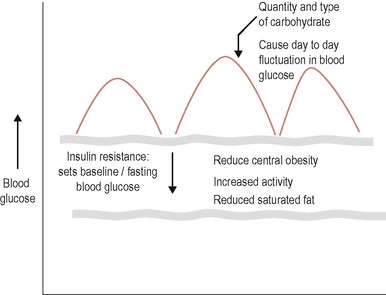
In dietary terms this means that all the messages related to insulin resistance will still be important, but in addition, for some people, the type and more specifically the amount of carbohydrate at any one time will also be important in determining day-to-day blood glucose excursions. This is represented diagrammatically in Figure 17.2.
In reality what this means is that at different stages of the development of diabetes, individuals may need to focus on differing aspects of nutritional therapy.
Insulin resistance will determine fasting blood glucose levels and is nutritionally affected by central obesity, saturated fat intake and physical activity. Oral agents such as metformin will also improve insulin sensitivity. All of these therapies will improve fasting and thus to some extent preprandial blood glucose levels.
Day-to-day excursions in blood glucose will occur when the carbohydrate load exceeds the capacity of the pancreas to produce enough insulin to deal with both underlying insulin resistance and the carbohydrate load of any meal or snack. This is more likely to be a problem as diabetes progresses and pancreatic failure necessitates the use of sulphonyurea or/and insulin treatment. In practice type 2 diabetes requires a ‘layering’ or stepping up of all treatments as the condition progresses. For lifestyle advice this means:
Initial advice. This is not necessarily provided by a dietitian:
• Avoid food and drink with a very high glycaemic index/load, e.g. glucose-based drinks, boiled sweets, jelly;
• No need to buy diabetic products (usually high in fat).
Step one. Dealing with insulin resistance:
• Avoid foods high in fat, especially saturated fat (including for that reason ‘diabetic’ products);
• Promote monounsaturated fats as an alternative to saturated fats, but be cautious about amount in overweight clients;
• Manage weight through portion size and calorie sources, such as alcohol, especially if weight loss is not achieved though lower fat and lower sugar choices;
• Promote physical activity.
Step two. Investigating day-to-day escalation in blood glucose levels:
• Consider carbohydrate portion and ‘glycaemic load’ of carbohydrate foods;
• Carbohydrate ‘awareness’ may be useful for some people on insulin therapy, who demonstrate variability in day-to-day blood glucose level.
Weight management
In type 2 diabetes, weight loss has been directly linked to mortality and will influence most cardiovascular risk factors as well as glycaemic management via improved insulin resistance. In 1990 Mike Lean demonstrated that for people with type 2 diabetes, at 12 months from diagnosis, each 1 kg of weight loss was associated with 3–4 months increased survival. 14 In terms of interim measures of risk, a 10% reduction in weight can result in a:
• 30–40% reduction in diabetes-related deaths;
• 15% reduction in HbA1c;
• 30–50% reduction in fasting glucose; and
• 10% reduction in total cholesterol.
Without doubt weight loss should be a ‘goal standard’ outcome in type 2 diabetes.
However, weight loss can also be a sign of poorly controlled diabetes. Glycosuria can cause ‘artificial’ weight loss if blood glucose levels are persistently above the renal threshold, (usually blood glucose levels of 10 mmol/L or more). All medical therapies that correct hyperglycaemia will cause indirect weight gain, through reduced glycosuria and associated calorie loss. With insulin therapy this has been found to be a 5-kg weight gain for a fall in HbA1c of 2.5%. 15 This means as medical therapies are commenced, small deficits in calorie intake should be negotiated to limit weight gain. For some individuals, weight maintenance might be a more realistic option.
Carbohydrate and Type 2 diabetes
Amounts of carbohydrate
There is currently no evidence to support carbohydrate counting in type 2 diabetes, even if insulin treated. It is inappropriate to suggest a ‘free’ diet, with flexible insulin as for type 1 diabetes. Previous dietary guidelines have promoted high intakes of complex carbohydrates, but concerns about weight gain, worsening post prandial glucose and deterioration in lipid profile (triglyceride) have lead dietitians to look at more modest portion control of carbohydrate. Table 17.3 lists the diet’s macronutrient components as suggested in the latest guidelines from the UK. 16 These recommendations allow for a higher proportion of monounsaturated fat to replace carbohydrate intake if clinically indicated.
| Component | Proportion |
|---|---|
| Protein | > 1 g per kg body weight |
| Total fat | < 35% of energy intake |
| Saturated and trans fat | < 10% of energy intake |
 | |
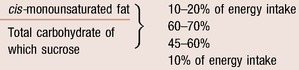 | |
There is no evidence to support the use of low carbohydrate diets (less than 130 g carbohydrate daily) as a management tool for blood glucose, and preliminary data from trials show no difference in outcome between low fat and low carbohydrate diets. The effect is mediated entirely by weight loss and indeed would indicate weight loss should be the primary nutrition therapy in type 2 diabetes. 16,17
Types of carbohydrates: glycaemic index
The glycaemic index (GI) has been a topic of much debate in diabetes. A recent meta-analysis of low glycaemic index diet trials in people with diabetes showed such diets produce a 0.4% reduction in HbA1c, if applied to individuals who had an initial high GI diet. 18
The GI has been useful for dispelling many misconceptions about carbohydrate foods. Research has shown that the ‘chain length’ of the carbohydrate has no effect on glycaemic response. This means that modest amounts of sucrose-containing products can be consumed, without deterioration in glycaemic control.
The GI of individual foods is mediated by a number of factors, such as the type of starch, the pectin content, cooking processes and the disruption of grains and starch granules, as outlined in Table 17.4. 18 In addition, other macronutrients consumed at the mealtime will also impact on GI. Co-ingestion of fat and protein tends to reduce the glycaemic response to a meal.
| Factor effecting GI | Mechanism | Example |
|---|---|---|
| Soluble fibre | Viscous fibres reduce rates of digestion by reducing enzyme action | Bean, oats, some fruit |
| Physical disruption of starch granules | Physical disruption of the starch granules provides greater surface area for digestion | Ground versus whole rice Whole fruit versus pureed fruit Puffed and extruded products |
| Gelatinisation of starches | Starch granules are heated using moist cooking methods, disrupting granules and increasing digestibility | Boiling potatoes |
| Resistant starch | Physically inaccessible starch, grain not physically disrupted, retrograded or ungelatinised starches | Wholegrains Cooled boiled potatoes Unripened bananas Partially cooked potato |
| Particle size of starch granules | Larger starch granules take longer to be digested | Pasta/rice slower than bread and potato |
| Amylose versus amylopectin | Amylopectin as a branch chain molecule has greater area for digestion | Some types of rice |
However, the reproducibility of glycaemic response to individual foods can be quite varied, particularly once applied to the mixed meal scenario, which makes it difficult to use in practice.
Because of the number of variables, the GI can be difficult to teach and can lead to mixed messages for people with type 2 diabetes. For example, many high fat foods have a low GI, such as cake and chocolate. These foods are clearly not good choices for people with type 2 diabetes. Many people also believe that high fibre will equate to low GI. This is not the case for foods rich in insoluble fibre such as wholemeal bread.
Practically, it may be helpful to promote low fat, low GI foods in place of higher fat foods or other sources of carbohydrate, for example, the use of wholegrain bread and cereals, legumes, oats and lower GI fruits.
All of this is unlikely to have an impact in itself and is simply fine tuning once other basic changes are in place, as outlined in the stepwise approach above. The exception to this is in pregnancy, where the post-prandial blood glucose is important to control and also in patients with type 1 diabetes using insulin pump therapy, where the infusion of mealtime insulin can be altered according to the glycaemic profile of the meal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree