Fig. 12.1
Panel (a) illustrates the pattern of spontaneous systemic metastases that can arise heterogeneously in mice within a month or more after surgical resection of an established primary orthotopic tumor using a metastatic variant (called LM2-4) selected in vivo from the MDA-MB-231 human breast cancer cell line. The majority of metastases are detected in the lungs but extrapulmonary metastases can sometimes also be found, e.g., in the liver and lymph nodes. Recurrence of tumor growth at the surgical resection site can also occur [23]. Panel (b) illustrates whole body bioluminescence imaging of LM2-4 tumors before primary tumor resection (“before”) or 5 or 30 days after mastectomy. Panel (a) is taken from Munoz et al. [23]; Panel (b) adapted from Ebos et al. [20]
Importantly, the variant metastatic cells can be easily tagged with an imageable biomarker, e.g., luciferase, enabling whole body optical bioluminescence imaging to monitor disease progression and therapeutic response of metastatic disease [20]. An example of the luciferase tagging is shown in Fig. 12.1b where orthotopic primary tumors are imaged in the upper panel and mice imaged 5 or 30 days after surgical resection of the primary tumors. This illustrates how initiating therapy within a few days of primary tumor resection would constitute a version of adjuvant therapy of early-stage (micrometastatic) disease in this model, whereas waiting a month after resection until initiating therapy would constitute treatment of overt metastatic disease. Examples are described below of how therapeutic outcomes can be very different depending on what stage of disease progression therapy is initiated. We have also utilized this serial selection approach to generate models of HER-2-positive metastatic breast cancer where mice are treated with a drug such as trastuzumab (Herceptin) [29, 30]. An obvious question is whether these metastatic models better reflect clinical outcomes compared to the conventional approach of treating established primary tumors. A number of examples follow which suggest this may be the case.
Emergence of Brain Metastases in Therapy-Induced Long-Term Survivors and Establishment of Spontaneous Brain Metastasizing Cell Lines
We recapitulated a common and important clinical therapeutic outcome of growing importance, namely, the emergence of spontaneous brain metastases in mice that had no evidence of such brain metastases when a successful treatment was first initiated in mice with systemic visceral metastases [27]. Presumably, as the result of extending the survival times of mice with such overt visceral metastatic disease, allowed what were asymptomatic microscopic brain metastases more time to develop into overt lesions [31, 32]. This brain metastasis “sanctuary” phenomenon, as it is known, is a discouraging observation in women with metastatic HER-2-positive cancer treated with trastuzumab-based therapy, i.e., they can experience a higher rate of relapse involving brain metastases [32]. The brain metastasis model we developed arose from treating mice with advanced systemic malignant melanoma with a chemotherapy protocol that extended survival [27]. When brain metastases were detected, they were isolated and cell lines were established from the lesions. The isolated variants were then analyzed for their ability to spontaneously metastasize to the brain from primary (and then resected) tumors after subdermal injection of the cells—but without any therapeutic intervention to extend survival. We found that, indeed, the cells were capable of such heritable spontaneous brain metastases [27]. Subsequent studies revealed possible molecular mediators of spontaneous metastasis, e.g., endothelins 1 and 3 and endothelin receptor B [33].
Recapitulating the Phase III Clinical Trial Failure of Anti-Angiogenic Drugs in Mouse Models with Metastasis
We undertook a comparative analysis of the therapeutic effects of an antiangiogenic drug—testing three different antiangiogenic agents—in the conventional circumstance of established primary orthotopic tumors, on the one hand, versus established visceral metastatic disease after primary tumor resection, on the other. An example of this is shown in Fig. 12.2. We found that oral anti-angiogenic TKIs such as sunitinib or pazopanib, as well as an antibody to mouse VEGFR-2 (called DC101), all caused primary antitumor growth delay. The results using sunitinib are shown in Fig. 12.2a (left panel) [34]. In contrast, no such efficacy was detected when treating overt metastatic disease (which was mainly confined to the lungs) as shown in Fig. 12.2a (right panel). Moreover, when sunitinib was combined with standard paclitaxel chemotherapy, again no efficacy was noted in the metastatic-treatment setting compared to other treatment groups, as also shown in Fig. 12.2b. These pre-clinical results, using the metastatic model, recapitulated the negative results of four failed randomized phase III clinical trials assessing sunitinib alone or combined with chemotherapy in women with metastatic breast cancer [35–38]. Furthermore, we also reported results that foreshadowed the subsequent failure of adjuvant anti-angiogenic therapy for the treatment of early-stage micrometastatic disease [20], including breast cancer, e.g., the adjuvant breast cancer “BEATRICE” [39], “BETH,” and ECOG-5103 adjuvant trials [21, 39]. This is shown in Fig. 12.3. Thus, as already described, treatment of mice with sunitinib was effective in the primary tumor treatment setting, as shown in Fig. 12.3a. However, in complete contrast when the primary LM2-4 breast cancer was resected and adjuvant treatment initiated immediately, using a short course of high dose sunitinib, the therapeutic outcome (as shown in Fig. 12.3b) was actually worse in the treated mice compared to the vehicle control [20].
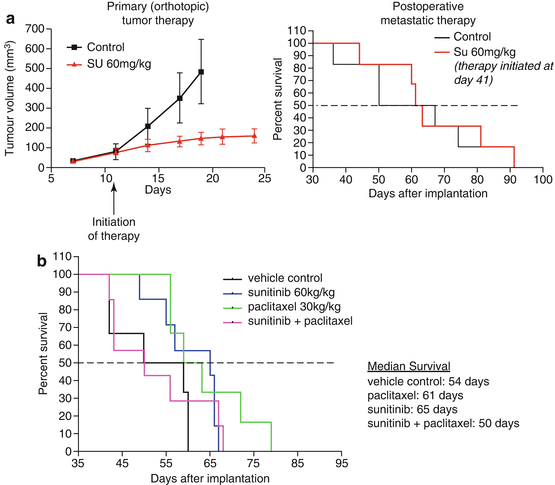
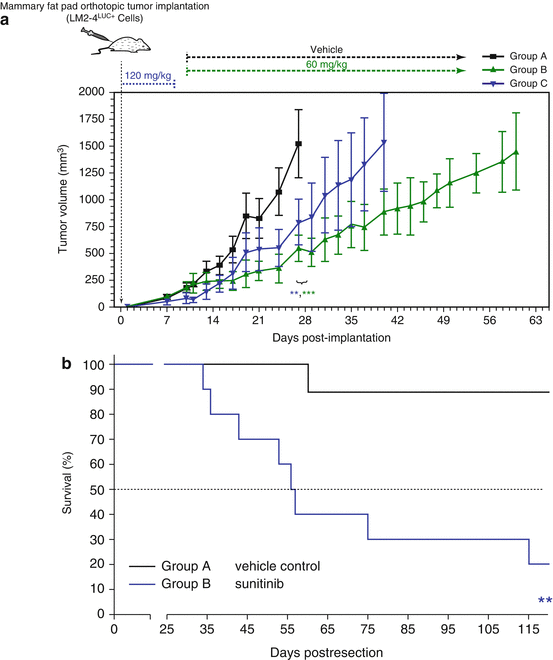

Fig. 12.2
Panel (a), left side, shows the positive efficacy impact of daily anti-angiogenic therapy using the anti-angiogenic TKI sunitinib on established orthotopic primary human breast cancer xenografts of the LM2-4 variant obtained from the MDA-MB-231 cell line, versus the lack of therapeutic impact (right side) of the same therapy when initiated in mice with established metastatic disease approximately a month after surgical resection of the primary tumor. Similar results to sunitinib were obtained with pazopanib and DC101, an anti-VEGFR-2 antibody when treating primary tumors. Panel (b) shows the therapeutic impact of sunitinib with paclitaxel chemotherapy in the metastatic setting where a lack of efficacy is observed in contrast to the primary tumor treatment setting. Taken from Guerin et al. [34]

Fig. 12.3
Panel (a) illustrates the therapeutic impact of sunitinib on established primary orthotopic breast cancer xenografts using the LM2-4 metastatic variant of MDA-MB-231 human breast cancer cells and two different doses and schedules of the drug. An antitumor benefit is caused by either protocol. In contrast a short course (1 week) therapy using the high dose of sunitinib (Group B) causes a decrease in survival in the postsurgical adjuvant treatment setting compared to Group A (vehicle control) where therapy was initiated a day after surgical resection of established primary tumors. Taken from Ebos et al. [20]
In the discussion of this published adjuvant therapy breast cancer study in 2009 [20], we cautioned that the clinical assessment of anti-angiogenic therapy in the adjuvant setting should perhaps be postponed until more information was known about the use of these agents in early-stage disease settings based on our pre-clinical results [20]. Subsequently, more than 12 randomized adjuvant trials involving bevacizumab plus chemotherapy or a TKI monotherapy have been undertaken in breast, colorectal, renal, lung and liver cancers, and all failed to meet their primary endpoint (reviewed in the Supplemental section of ref. 21). Although none showed a worse outcome with one exception (a colorectal trial called AVANT), it is notable that all the bevacizumab trials involved bevacizumab plus chemotherapy followed by maintenance bevacizumab. It is thus possible that the addition of chemotherapy may have prevented any potential pro-invasive/metastatic effect induced by the anti-angiogenic drug treatment. Indeed, we recently published evidence in tentative support of this hypothesis. In brief, we found that DC101 (a VEGFR-2 antibody) or sunitinib could increase local invasion of orthotopic primary breast tumors, and moreover, this undesirable effect could be prevented by the addition of concurrent chemotherapy, using either paclitaxel or cyclophosphamide [40], as shown in Fig. 12.4. In this same study, we also showed that a sequenced combination of neoadjuvant followed by adjuvant anti-angiogenic-based therapy could bring about an overall survival effect (shown in Fig. 12.5). Interestingly, this limited study result recapitulates a secondary analysis of a recent adjuvant Phase III clinical trial outcome in breast cancer (NSABP-B-40) involving neoadjuvant chemotherapy followed by adjuvant bevacizumab therapy, showing an overall survival benefit [41].
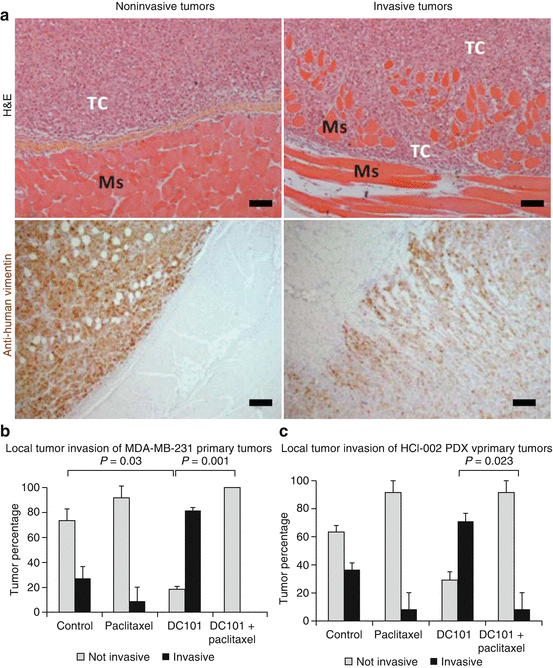
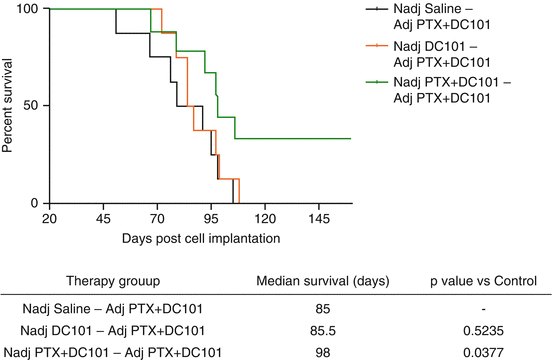

Fig. 12.4
Increase in local invasion after anti-angiogenic therapy and blockade of this effect by concurrent chemotherapy. MDA-MB-231 breast cancer cells and the triple-negative PDX breast cancer HCI-002 were orthotopically implanted in the mammary fat pads of SCID mice. When tumor volumes reached 150 mm3, therapy was started with vehicle, DC101 (a VEGFR-2 antibody), paclitaxel (30 mg/kg once every 2 weeks), or the combination of the two. (a) Tumors are considered invasive when tumor cells encroached into the adjacent muscular fibers of the abdominal wall. Left panels show a noninvasive MDA-MB-231 tumor stained for H&E (upper panel) and human vimentin antibody (lower panel, cells in brown). Right panels show invasive tumors stained for H&E (upper panel) and anti-human vimentin (lower panel). Ms muscle cells of the abdominal wall, TC tumor cells. Scale bars, 150 μm. (b, c). Graphs showing the percentage of invasive and noninvasive tumors in MDA-MD-231 (b) and PDX HCI-002 (c) breast tumors. *p < 0.05 by Mann-Whitney test. Error bars indicate ±SD. In all groups n ≥ 6. Results taken from Paez-Ribes et al. [40]

Fig. 12.5
Neoadjuvant + adjuvant therapy with DC101, the VEGFR-2 antibody, and paclitaxel increases survival and reduces toxicity. Mice were implanted with the metastatic variant LM2-4, and therapy was started when tumor volumes reached 150 mm3 and maintained for 10 days [DC101 (800 μg twice a week)] or the combination of DC101 and paclitaxel (30 mg/kg every other week). Tumors were resected when they reached 500 mm3, and 5 days later therapy with DC101 and paclitaxel protocol was started in all mice for 4 weeks. The Kaplan-Meier survival curve shows the overall survival of mice after receiving neoadjuvant + adjuvant therapy. The log-rank test was used for statistics. Taken from Paez-Ribes et al. [40]. This result superficially recapitulates the secondary analysis of an overall survival benefit in the NSABP-B-40 neoadjuvant/adjuvant trial of bevacizumab plus chemotherapy in early-stage breast cancer [41]
Comparing Orthotopic Primary Tumors vs. Spontaneous Metastases Reveals Evidence for Non-angiogenic “Vessel Co-option” in Lung Metastases
With these previous published anti-angiogenic therapy results in mind, we subsequently focused on another important question using our orthotopic primary tumor or postsurgical human tumor xenograft models. We asked why it is that the primary orthotopic breast cancers in our studies are sensitive to an anti-angiogenic drug treatment, whereas distant metastases, found mainly in the lungs, are apparently not. To address this question, we analyzed the nature of the tumor vasculature populating primary tumors versus lung metastases and did so in collaboration with several investigators—Dr. Harold Dvorak in Boston; Dr. Andrew Reynolds in London, UK; and Dr. Peter Vermeulen in Antwerp, Belgium [42]. We found that whereas the primary tumors are highly angiogenic, based on histopathologic criteria, the lung metastases are not. Instead, as shown in Fig. 12.6, we found evidence of what is known as “vessel co-option.” This refers to the property of tumor cells to hijack, i.e., co-opt, the existing abundant vasculature in a particular organ site such as the lungs. For example, the alveolar spaces/air sacs can become filled with cancer cells and simply utilize the existing extensive blood vessel network in the membranes surrounding the air sacs [42], or alternatively, the cancer cells can “cuff” the membranes and parasitize their existing blood vessels. In either circumstance, as tumors in the lungs grow and expand, there is little or even no need to induce new blood vessel formation, i.e., sprouting neoangiogenesis. Previous pre-clinical work by Szabo et al. also showed lack of angiogenesis in lung metastases, where vessel co-option was dominant in a variety of lung metastasis models in mice [43].
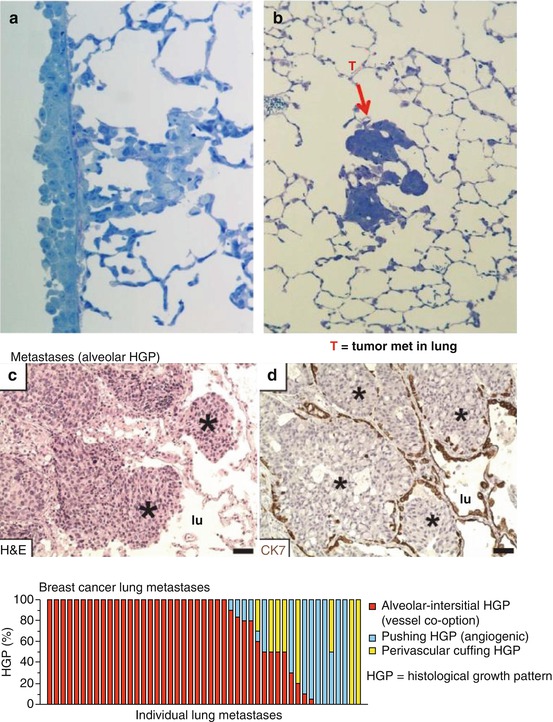

Fig. 12.6
(a) illustrates “cuffing” of tumor cells around the membranes surrounding lung alveolar air sacs of spontaneous lung metastases in the LM2-4 metastatic model, while (b) shows metastatic tumor (T) cells of a spontaneous polyoma middle T-driven breast cancer “GEMM” filling up the air sacs. (c, d) illustrate vessel co-option in human breast cancer lung metastases, and the bottom panel illustrates the extent of such vessel co-option detected in multiple, individual human breast cancer metastases (red bars in bottom panel). (c, d) and bottom panel taken from Bridgeman et al. [42]. * indicates aveolar sacs filled with cancer cells
Vessel co-option was first reported in the modern literature in 1996 by Francesco Pezzella and colleagues when studying human non-small cell lung cancer [44, 45]. Both primary tumors and metastases growing in lung tissue often show minimal or no sprouting angiogenesis. Instead, they show evidence of vessel co-option. These observations have been confirmed by an examination of over 164 human lung metastasis cases, including cases of lung metastasis derived from breast, colorectal, and renal cancer patients [42]. As such, this could readily explain the modest effects or even the absence of a therapeutic effect when utilizing an anti-angiogenic drug to treat lung metastases. Moreover, vessel co-option has also been described in liver metastases of colorectal cancer by Peter Vermeulen and colleagues [46].
The vessel co-option results described above could readily account for some of the modest effects of anti-angiogenic drugs, in general, and for treatment of metastatic breast cancer in particular. Moreover, they may also account for some instances of acquired/evasive resistance to such drugs. Indeed, we recently published results utilizing a model of orthotopic HCC using the anti-angiogenic TKI called sorafenib [47]—which is approved for HCC patients—showing that it is initially effective in blunting the growth of the HCC tumors during which time tumor angiogenesis can be detected. However, within about a month, the sorafenib treatment begins to lose its efficacy, and this coincides with a switch by the tumors from relying on angiogenesis to exploiting vessel co-option. The switch to vessel co-option is preceded by a marked invasion (migration) of the sorafenib-treated cancer cells into the liver and eventually replacing the liver parenchyma so that the tumor comes to co-opt the pre-existing liver vasculature [47]. Of considerable interest is that similar results showing a switch to co-option was reported clinically in liver metastases of colorectal cancer patients receiving a bevacizumab-based therapy [48].
These vessel co-option results have raised a critical question, namely, can co-opted blood vessels in tumors be therapeutically targeted in a safe manner by an anti-vascular therapy that is independent of inhibiting angiogenesis? If so, this could open up a new therapeutic era involving targeting of the tumor vasculature. In this regard, there is a basis for speculating that co-opted tumor vessels are not completely normal. For example, blood vessels in metastatic sites often show evidence of hyper-permeability, i.e., “vascular leak,” and this physiologic abnormality along with their structural abnormalities (e.g., as a result of extensive cancer cell compression and high interstitial fluid pressures) and the fact that they are enveloped by cancer cells secreting various growth factors, cytokines, and enzymes make it likely that they express various abnormalities. These abnormalities may, in some cases, be therapeutically exploited so as to render them vulnerable while sparing the normal (tumor-free) vasculature in the same organ or other organs/tissues elsewhere in the body.
Studies with Patient-Derived Xenografts (PDXs)
All of the aforementioned work, as well as other studies not summarized here involving human orthotopic and metastatic colorectal cancer [24] and renal cell carcinoma [25] xenografts, involved the use of long-term-established human cell lines. We asked whether the basic approach could be adapted and extended to PDXs by evaluating whether spontaneous metastasis PDX models could be developed utilizing a similar approach for successfully establishing such models using established cell lines [2]. We also undertook some comparisons of the response to a particular therapy using primary tumors, established from human tumor cell lines on the one hand, versus PDXs on the other [49]. With respect to the first initiative, the results were disappointing. We analyzed several different triple-negative human breast cancer PDXs, e.g., HCI-001 and HCI-002, among three others, that were obtained from Dr. Alana Welm, Huntsman Cancer Institute, Utah [50]. The tumors were orthotopically transplanted and then surgically resected. A total of 144 SCID mice were used as recipients for these tumor transplantations.
Unfortunately detection of distant overt metastases visible upon gross inspection was exceedingly rare with only three lung metastases detected among the 144 transplanted mice [6]. The protocol used and results are summarized in Fig. 12.7 and Table 12.1. Moreover, when a metastasis was transplanted orthotopically as a histologically intact tissue mass, no evidence of increased metastatic aggressiveness was noted—in marked contrast to the results we obtained using an established human tumor cell line, e.g., MDA-MB-231, to select metastatic variants [23]. Although a number of tumors outside the transplant site were noted, they turned out, with rare exception, to be de novo mouse thymomas [6]. The reasons for the failure to obtain distant overt metastatic disease using PDXs in this study are unknown, but several possibilities come to mind. For example, we used SCID mice as the recipients for these studies and it is known that mice with greater degrees of immune deficiency such as NOD-SCID-IL-2γR−/− NSG/NOG mice are more prone to developing metastatic disease (reviewed in ref. 50). Indeed, we undertook a previous comparative analysis of the metastatic aggressiveness of MDA-MB-231 cells and the LM2-4 variant selected from MDA-MB-231 in SCID, NOD-SCID, and NSG mice after orthotopic injection of tumor cells followed by surgical resection of the primary tumors [51]. The results highlighted the much greater metastatic aggressiveness in NSG mice [51]. In fact, even the “poorly metastatic” parental MDA-MB-231 tumor cell line was highly metastatic in NSG mice which suggests metastatic spread may be strongly restricted by cellular elements of the innate immune system that are missing or deficient in the NSG mice [51]. In addition, most PDXs are obtained from primary tumors or malignant effusions, as opposed to distant isolated metastases. Using PDXs derived from metastases may result in a greater chance of detecting distant metastases in recipient mice. What is intriguing about our preliminary inability to develop models of metastatic disease, or to detect metastases with any frequency using PDXs, is that these results stand in marked contrast to an impressive body of literature from the group led by Robert Hoffman reporting that surgical orthotopic implantation (SOI) to develop “PDOXs” (patient-derived orthotopic xenografts) resulted in clinically relevant patterns of metastatic disease when studying a very broad spectrum of human tumor types [11–19] as cited earlier. Moreover, these experiments were mostly undertaken using athymic nude mice as recipients. The reasons for the discrepancy in these results and ours remain to be determined but may reveal important factors involved in metastatic efficiency in addition to host-mediated control mechanisms of metastatic disease and responsiveness to therapy [6]. We should also acknowledge some recent results by the group of Dr. Zena Werb, i.e., Lawson et al, “Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells” Nature 526:131–135, 2015, which would appear to be a variance with ours. These authors reported that by using molecular markers such as antibodies to human CD298 (ATP1B3) for immunohistochemistry or FACS based assays, that metastatic PDX-derived cells could be detected in peripheral tissues (e.g. lung, liver, brain, bone, marrow, lymph node) in 70% of mice that had undergone surgical resection of a primary orthotopic PDX breast cancer. NOD-SCID mice showed evidence of either “low burden” or “high burden” disease, though high burden disease seemed to consist of microscopic or small nodules in greater numbers. Thus imaging and detailed molecular detection may reveal evidence of spontaneous metastatic disease using PDXs; however, the extent of overt life-threatening overt metastatic disease that develops in this study remains unclear.
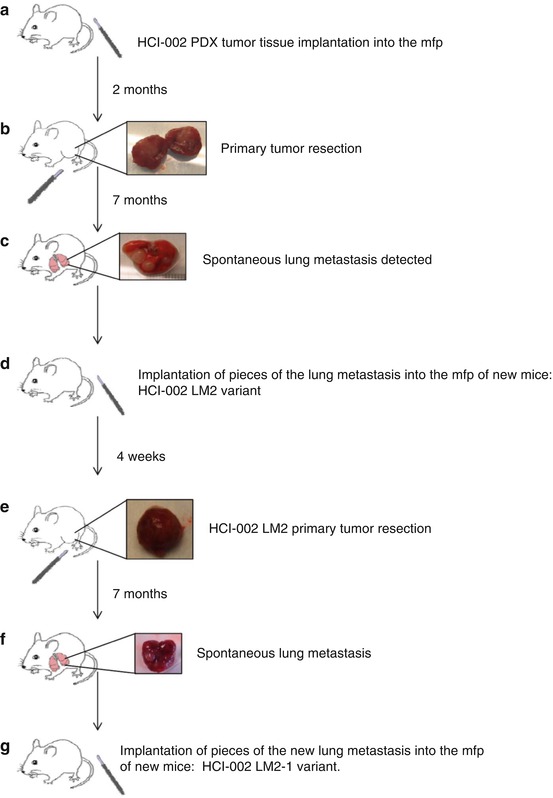

Fig. 12.7
Flow diagram of methodology used to try and generate spontaneous metastases from a primary resected breast cancer PDX called HCI-002. mfp mammary fat pad. Taken from Paez-Ribes et al. [6]
Table 12.1
Summary of secondary tumor detection after orthotopic primary tumor resection detected in SCID mice transplanted with triple-negative human breast cancer PDXs
Tumor type | Metastases of human origin | Tumors of mouse origin (%)a | Total mice implanted |
|---|---|---|---|
HCI-001 | 1 | 6 (15.3) | 39 |
HCI-002 | 2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|