Tumor type
Tumor tissue median pO2 mm Hg (patients)
Normal tissue median pO2 mm Hg
Pancreas
2 (8 pts)
57
Brain
13 (104 pts)
26
Head and Neck
10 (592 pts)
n/a
Lung
16 (26 pts)
n/a
Breast
10 (212 pts)
52
Cervix
9 (730 pts)
42
Liver
6 (4 pts)
30
Sarcoma
14 (283 pts)
51
Melanoma
12 (18 pts)
41
17.3 Antitumor Drugs and Hypoxia
Chemotherapy failure comes from the three basic categories; inadequate pharmacokinetic properties of the drug tumor cell internal factors, expressed as drug efflux pumps and the extrinsic condition of the tumor cell microenvironment, including hypoxia, acidosis, nutrient starvation, and increased interstitial pressure. It is known that tumor hypoxia affects therapy outcome negatively. Hypoxia decreases tumor cell proliferation and promotes cell cycle arrest. Ultimately, hypoxia is derived from chemoresistance, because anticancer drugs predominantly target rapidly proliferating cells. When screening antiproliferative substances in vitro, this knowledge has been ignored, resulting in the failure of developing novel anticancer drugs in hypoxia condition in vivo. In tumors, large areas of hypoxic microenvironment often exist, and these are related to a poor prognosis. These regions of massive hypoxia in tumor are thought to be resistant to the treatment of chemotherapy and radiotherapy. Regarding traditional older types of chemotherapy, poor tissue penetration and targeting were due to a more rapid wash-out from regions of tumors, that are located to the tumor vascularity.
The critical unmet medical needs that exist with focus on hypoxic tumors and novel therapeutics, that selectively target hypoxic tumor cells, have to be developed clinically. Aggressive efforts of prodrugs, which are highly and selectively activated in hypoxic regions, began with the nitrobenzyl systems over a quarter century ago [16]. Recently, the hypoxia region activated prodrug, tirapazamine, is in a clinically advanced stage. Many different solid tumors have been investigated in phase III clinical trials of tirapazamine; however, the results have been disappointing due to poor tumor penetration and low in vivo potency at tolerated doses [17]. Hypoxia-activated phosphoramidate DNA cross-linking mustards were introduced by Borch et al. Among the most successful prodrugs were 5-nitrothiophene- and 5-nitrofuran-triggered prodrugs of phosphoramidate toxins. This active form was based on cyclophosphamide, a commonly used antitumor drug. Isophosphoramide mustard is the prodrug of ifosfamide, a clinically useful anticancer agent, and it is the cytotoxin generated from the cytochrome P450 activation. Its clinical efficacy has been proven in trials [18].
17.4 A Promising Prodrug
TH-302 (Threshold Pharmaceuticals, San Francisco) (1-methyl-2-nitro-1H-imidazole-5-yl) N, N0-bis (2-bromoethyl) diaminophosphate is defined as a 2-nitroimidazole-linked prodrug of a brominated version of isophosphoramide mustard (Br-IPM) [19]. Bioreductive enzymes reduce one-electron from TH-302, thereby generating a free radical anion. Under aerobic conditions, these anions are reported to be restored to their original state, and are induced to the production of superoxide via redox cycling. TH-302 has shown almost no cytotoxic activity under normoxic conditions, and massively augmented cytotoxic activity under hypoxic conditions in terms of in vitro cytotoxicity and clonogenic assays using human cancer cell lines [16, 20]. Both nondividing and dividing cells are killed by a bis-alkylator, acting as a DNA cross-linking agent. “S139 phosphorylation of histone H2AX is thought to ultimately kill tumor cells, when the Br-IPM moiety is efficiently activated and induces intramolecular cross-linkage of DNA” [21–23]. TH-302 exhibited antitumor activity in a dose-dependent fashion and strongly correlated with total drug exposure. Association between its antitumor activity and tumor hypoxic conditions was found across 11 xenograft models. Animals with tumor breathing 95% O2 showed a decrease of TH-302 efficacy, while on the other hand those breathing 10% O2 showed significantly enhanced TH-302 efficacy, both compared with breathing of air (21% O2). Treatment with TH-302 resulted in reduction in accordnace with the tumor hypoxic conditions and a related increase in the necrotic region. Interestingly, DNA damage introduced by TH-302 as measured by gH2AX was initially only observed in the hypoxic regions, and then expanded to the entire tumor in a time-dependent fashion. This evidence from preclinical experiments has strongly demonstrated a bystander effect of TH-302, which may be related to the diffusion of the active form to adjacent normoxic regions of tumor, and strongly indicated an additional and significant antitumor activity. Since TH-302 is preferentially activated in hypoxic tissues and does not have the same toxic metabolites as ifosfamide following hepatic metabolism, it is considered that TH-302 may show fewer drug-related adverse effects than ifosfamide. This hypothesis might be supported by the reversible skin and mucosal toxicities observed to be dose-limiting factors in a phase I clinical trial of HT-302 [24].
17.5 Clinical Development
Several clinical trials have been performed during last decades, in which the target molecule against hypoxia was HIF-1. A phase I clinical trial of topotecan, an FDA-approved semisynthetic camptothecin analogue, has been opened to patient enrollment. Topotecan is thus supposed to be the first HIF-1 inhibitor to be tried in humans. The second HIF-1 inhibitor likely to enter the clinic might be PX-478, from ProlX Pharmaceuticals in Tucson, Ariz; however, excellent clinical effect targeting hypoxia has not yet obtained for this drug. On the other hand, clinical trials of several prodrugs activated in hypoxia tissue have also been performed.
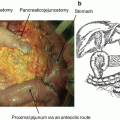
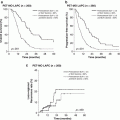
In particular, TH-302 has shown preclinically and clinically strong antitumor activity in a variety of solid tumors, including pancreatic cancer [22, 25, 26]. In a phase I/II clinical study against solid tumors, investigating TH-302 doses of 240–575 mg/m2 on days 1, 8, and 15 of a 28-day cycle, the recommended phase II dose of the combination of gemcitabine with TH-302 was established as 340 mg/m2. Dose-limiting hematologic and mucosal toxicities were more frequent at 340 than 240 mg/m2. The overall response rate was 21%, and median PFS time was 5.9 months when observed in 46 patients with advanced pancreatic cancer [26]. To evaluate the benefit of combination TH-302 to gemcitabine, an open-label, multicenter, randomized phase II clinical trial was designed and conducted, as systemic therapy in patients with previously untreated advanced pancreatic cancer, as the standard of care at the time, namely first-line chemotherapy against advanced pancreatic cancer.
The randomized phase II study was performed at the 45 sites. A total of 214 subjects with advanced pancreatic cancer were enrolled and randomized to three groups: Gemcitabine (1000 mg/m2) alone, Gemcitabine + TH-302 (240 mg/m2), and Gemcitabine + TH-302 (340 mg/m2) group. Crossover was permitted from 240 to 340 mg/m2 of TH-302 plus Gemcitabine. The primary end points were PFS and Safety. Secondary end points were response rate by RECIST 1.1; change in CA19-9 including CA19-9 response (>50% decrease); and overall survival. Table 17.2 shows the clinical effect of this phase II trial [11]. Median of PFS values in this trail were as follows: Gemcitabine alone group, 3.6 months (N = 69); Gemcitabine + TH-302 240 mg/m2 group, 5.6 months (N = 71); and Gemcitabine + TH-302 340 mg/m2 group, 6.9 months (N = 74). Hazard ratio (HR) vs Gemcitabine alone group was 0.655 (95% CI: 0.46–1.02) and p = 0.060 (Log-rank test) in Gemcitabine + TH302 240 mg/m2 group, and 0.589 (95% CI: 0.4–0.88), p = 0.008 (Log-rank test) in Gemcitabine + TH302 340 mg/m2 group, respectively. RECIST best response was as follows: CR and PR in Gemcitabine alone group were 0% and 12% in Gemcitabine + TH-302 240 mg/m2 group were 0% and 17% and in Gemcitabine + TH-302 340 mg/m2 group were 3% and 23%, respectively. P-value of Gemcitabine + TH302 240 mg/m2 vs Gemcitabine alone was 0.22, and that of Gemcitabine + TH302 340 mg/m2 vs Gemcitabine alone was 0.021. Maximum decrease and response of CA19–9 was as follows: mean nadir change (U/L) in CA19–9, Gemcitabine alone group, −523; Gemcitabine + TH302 240 mg/m2 group, −3909; and Gemcitabine + TH302 340 mg/m2 group, −5385. Percent CA 19–9 Decrease >90% was as follows: Gemcitabine alone group, 16%; Gemcitabine + TH302 240 mg/m2 group, 24%; and Gemcitabine + TH302 340 mg/m2 group, 32%. Median OS was as follows: Gemcitabine alone group, 6.9 months; Gemcitabine + TH302 240 mg/m2 group, 8.7 months; and Gemcitabine + TH302 340 mg/m2 group, 9.2 months. HR of TH302 240 mg/m2 group vs Gemcitabine alone group was 0.960 (95% CI: 0.67–1.38) and p = 0.827 (Log-rank test), and that of TH302 340 mg/m2 group vs Gemcitabine alone group was 0.955 (95% CI: 0.67–01.37) and p = 0.800(Log-rank test). Survival at 6 and 12 months by treatment arm was as follows: 6-month survival of Gemcitabine alone group, Gemcitabine + TH302 240 mg/m2 group, and Gemcitabine + TH302 340 mg/m2 group were 57%, 69%, and 73%, respectively. P-value of Gemcitabine + TH302 240 mg/m2 group vs Gemcitabine alone group was 0.123, and that of Gemcitabine + TH302 340 mg/m2 group vs Gemcitabine alone group was 0.037. Moreover, 12-month survival of Gemcitabine alone group, Gemcitabine + TH302 240 mg/m2 group, and Gemcitabine + TH302 340 mg/m2 group were 26%, 37%, and 38%, respectively. P-value of Gemcitabine + TH302 240 mg/m2 group vs Gemcitabine alone group was 0.178, and that of Gemcitabine + TH302 340 mg/m2 group vs Gemcitabine alone group was 0.130. Longer survival after crossover randomization was achieved from 2.6 months to 13.4 months [11]. In terms of safety, there was a decrease in study discontinuations due to AEs from 16% to 12%, in Gemcitabine alone group as compared with Gemcitabine + TH302 340 mg/m2 group, respectively. In summary it was strongly expected that clinical efficacy would be promising and it showed strong signals for clinical benefits [11].
Table 17.2
Clinical response
Response | Gemcitabine alone | G + T240 | G + T340 | |||
|---|---|---|---|---|---|---|
No. of patients | % | No. of patients | % | No. of patients | %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |