AE Term |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Alopecia |
Hair loss of < 50% of normal for that individual that is not obvious from a distance but only on close inspection; a different hair style may be required to cover the hair loss, but it does not require a wig to camouflage |
Hair loss of ≥50% normal for that individual that is readily apparent to others; a wig is necessary to completely camouflage the hair loss; associated with psychosocial impact |
|
|
|
Dry skin |
Covering < 10% BSA and no associated erythema or pruritus |
Covering 10-30% BSA and associated with erythema or pruritus; limit instrumental ADL |
Covering > 30% BSA and associated with pruritus; limiting self-care ADL |
|
|
Hypertrichosis |
Increase in length, thickness, or density of hair that the patient is either able to camouflage by periodic shaving or removal of hairs or is not concerned enough about the overgrowth to use any form of hair removal |
Increase in length, thickness, or density of hair at least on the usual exposed areas of the body that requires shaving or use of hair removal to camouflage; associated with psychosocial impact |
|
|
|
Mucositis |
Asymptomatic or mild symptoms; intervention not indicated |
Moderate pain; not interfering with oral intake; modified diet indicated |
Severe pain; interfering with oral intake |
Life-threatening consequences; urgent intervention indicated |
Death |
Palmar-plantar erythrodysesthesia syndrome |
Minimal skin changes or dermatitis (e.g., erythema, edema, and hyperkeratosis) without pain |
Skin changes (e.g., peeling, blisters, bleeding, edema, and hyperkeratosis) with pain; limiting instrumental ADL |
Severe skin changes with pain; limiting selfcare ADL |
|
|
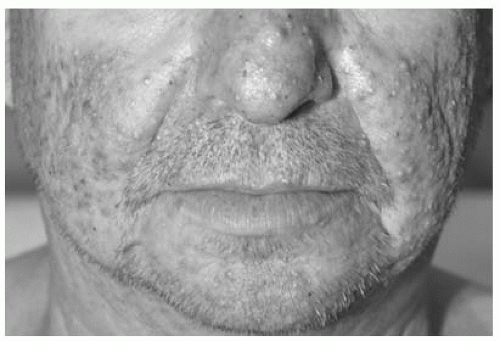
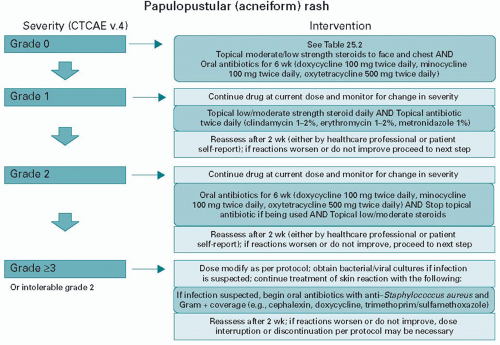
Papulopustular rash |
Papules and/or pustules covering < 10% BSA, which may or may not be associated with symptoms of pruritus or tenderness |
Papules and/or pustules covering 10-30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; associated with psychosocial impact; limiting instrumental ADL |
Papules and/or pustules covering > 30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; limiting selfcare ADL; associated with local superinfection with oral antibiotics indicated |
Papules and/or pustules covering any % BSA, which may or may not be associated with symptoms of pruritus or tenderness and IV antibiotics indicated; life-threatening consequences |
Death |
Paronychia |
Nail fold edema or erythema; disruption of the cuticle |
Localized or oral intervention indicated; edema or erythema with pain; associated discharge or nail plate separation; limiting instrumental ADL |
Surgical intervention or IV antibiotics indicated; limiting self-care ADL |
|
|
Pruritus |
Mild or localized; topical intervention indicated |
Intense or widespread; intermittent; skin changes from scratching; oral intervention indicated; limiting instrumental ADL |
Intense or widespread; constant; limiting selfcare ADL or sleep; oral corticosteroid or immunosuppressive therapy indicated |
|
|
Radiation dermatitis |
Faint erythema or dry desquamation |
Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema |
Moist desquamation in areas other than skin folds and creases; bleeding induced by minor trauma or abrasion |
Life-threatening consequences; skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site; skin graft indicated |
|
Rash maculopapular |
Macules/papules covering < 10% BSA with or without symptoms (e.g., pruritus, burning, and tightness) |
Macules/papules covering 10-30% BSA with or without symptoms; limiting instrumental ADL |
Macules/papules covering > 30% BSA with or without symptoms; limiting self-care ADL |
|
|
AE, adverse event; BSA, body surface area; ADL, activities of daily living. |