Highlights
- •
The most common definition of delayed DTI is a time interval exceeding 30 days.
- •
Delays in DTI over 30 days significantly reduce overall survival for head and neck cancer patients.
- •
Effects of delayed DTI on quality of life remain underexplored and need further research.
Abstract
Introduction
Delayed diagnosis to treatment interval (DTI) in head and neck cancers (HNC) can significantly impact patient outcomes. The need for multimodal treatment in HNC may cause delays in initiation of treatment. This systematic review aims to provide a comprehensive understanding of the consequences of delayed DTI on both oncologic and QoL outcomes, proposing a new quality benchmark along the treatment continuum of HNC patients.
Methods
We searched MEDLINE, Embase, CENTRAL, Web of Science, and CINAHL databases for trials and cohort studies evaluating the impact of delayed DTI in patients with HNC. Outcomes included overall survival (OS), disease-free survival (DFS), locoregional (LRR) and local recurrences (LR) and distant metastasis.
Results
Our search strategy initially identified 10,779 titles and abstracts, of which 63 articles met inclusion criteria for a total of 873,718 patients. The pooled analysis showed that treatment initiation within 30 days improved OS by 9 % compared to longer intervals (aHR: 1.09 [1.06–1.13]; I 2 = 80 %), with no significant associations found for DFS, LRR, LR, or distant metastasis.
Conclusion
While adherence to a 30-day DTI may be associated with improved survival in some HNC patients, significant heterogeneity in the data limits the generalizability of this finding. Further research with more refined analyses, including adjustments for treatment modality and cancer stage, is necessary. Additionally, gaps remain in the literature, particularly in the methodological limitations related to DTI classification.
Introduction
Delays in cancer treatment have been associated with poorer outcomes, particularly in cancers like breast, bladder, colon, and head and neck (HNC) . In HNC, treatment efficiency can be assessed through various intervals, including the diagnosis to treatment initiation (DTI) interval, the Surgery to PostOperative Radiotherapy interval (S-PORT), and a composite interval from the surgery to the end of radiotherapy – treatment package time (TPT). The need for complex multidisciplinary multimodal treatment coordination in HNC’s may be a source of delays . Currently, the Commission on Cancer (CoC) sets a single quality metric for HNC, advocating for adjuvant postoperative radiotherapy (S-PORT) to start within six weeks post-surgery . However, evidence shows that nearly half of patients do not meet this standard, indicating a discrepancy in adherence and the need for solid scientific evidence to support future guidelines and metrics . Furthermore, the impact of delaying other intervals, such as the diagnosis to treatment interval (DTI), is less well studied. This systematic review aims to provide a comprehensive understanding of the consequences of delayed DTI on oncologic outcomes, proposing a new quality benchmark for the treatment continuum of HNC patients.
Methods
Study design
This is a systematic review with meta -analysis of cohort studies and clinical trials. The research protocol was registered with the international prospective register of systematic reviews platform, PROSPERO [CRD42024528247], and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines .
Information sources and search strategy
Key databases, including MEDLINE, EMBASE, Web of Science, Cumulative Index to Nursing & Allied Health Literature (CINAHL), and CENTRAL, were systematically searched from their inception to October 2023 to identify peer-reviewed journal articles and conference abstracts assessing the impact of delayed DTI on the prognosis of patients with aerodigestive HNC. In collaboration with an information specialist (M. R.) Medical Subject Headings (MeSH), Emtree words, CINAHL descriptors, and free text words, were employed to conduct the literature search. A sample search strategy can be found in the Supplementary material s ( Table S1 ). Forward searches through the bibliographies of all included studies and previous systematic reviews were conducted to ensure inclusion of relevant publications. Conference proceedings from the preceding three years (2020 to 2023) from key conferences, including the American Head & Neck Society (AHNS), the European Head & Neck Society, the American Association of Otolaryngology and Head & Neck Society, the Combined Otolaryngology Spring Meetings (COSM), the Canadian Society of Otolaryngology – Head and Neck Surgery (CSO-HNS), and the International Federation of Otorhinolaryngological Societies (IFOS), were also reviewed.
Study eligibility and selection
Studies that evaluated the impact of treatment delays on survival in patients with HNC of the aerodigestive tract were included. Case reports and case series (N ≤ 5) were excluded. Studies including populations of adult patients (age ≥ 18 years) with squamous cell carcinoma (SCC) of the aerodigestive tract head and neck sites (i.e. the oral cavity, oropharynx, hypopharynx, larynx, nasopharynx, and sinonasal regions) were selected. The specific inclusion criteria of this study were:
- 1.
Analysis of DTI and its impact on any of the prespecified outcomes;
- 2.
Curative intent treatment consisting of surgery followed by radiotherapy, or surgery followed by concurrent chemoradiotherapy;
- 3.
Squamous cell carcinoma pathologic variant of HNC
Studies were excluded if over 10 % of the study cohort included non-SCC histopathologic subtypes. Language restrictions for the inclusion of studies were not applied. Two reviewers (N.V.P. and K.G.) independently screened studies for inclusion, retrieved potentially relevant studies, and judged on final eligibility. Disagreements were resolved by a third reviewer (A.E.), however, an inclusive approach was employed at this review step. References retrieved from search strategies were stored in EndNote 21 and uploaded to Covidence (Covidence Systematic Review Software, Australia) where automatic deduplication was performed. Covidence was also used for the selection process.
Outcome measures
The primary outcome was overall survival (OS). Secondary outcomes included disease-free survival (DFS), locoregional recurrences (LRR), local recurrences (LR) and distant metastasis. For any outcome, the 5-year endpoint or the latest assessment when reported at different timepoints were used.
Data extraction
Two independent reviewers (N.V.P. and K.G.) extracted study-level data using a standardized and pretested data extraction form. The extracted data encompassed study characteristics, patient demographics, disease features (anatomic site, stage, etc.), treatment characteristics (treatment modality, systemic therapy, frequency, and definition of delays), and primary and secondary outcomes. Data from graphical presentations of outcomes were extracted using WebPlotDigitizer software (Version 4.1, San Francisco, CA, USA) .
Risk of bias assessment, certainty of evidence, and strength of recommendations
Risk of bias was evaluated independently by two authors (N.V.P. and K.G.) using the ROBINS-I tool (Risk of Bias in Non-randomized Studies–of Interventions) for observational studies . Disagreements were resolved by a third reviewer (A.E.). The certainty of evidence and strength of recommendations was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system. The final quality of evidence was classified as high, moderate, low, or very low for each clinical outcome.
Data synthesis and analysis
For each outcome of interest, data from individual studies were pooled to estimate overall pooled effects using the Der Simonian and Laird random effect model. Heterogeneity among studies was assessed using the I 2 test. Heterogeneity was considered significant for an I 2 value greater than 50 % . For each outcome of interest, we pooled the adjusted hazard ratio (aHR) from individual studies if more than 2 studies had reported such data and all these studies were deemed to have at least moderate quality of evidence using the ROBINS-I tool. We required the aHR estimates to account for at least patient age and sex as well as cancer stage. If more than one eligible aHR estimates were reported by a single study, we only extracted the one that was the most adjusted and encompassed the largest study cohort. Subgroup analyses were conducted to evaluate sources of heterogeneity for the primary and secondary outcomes. Prespecified subgroups included specific anatomic cancer sites (oral cavity, oropharynx, hypopharynx, and larynx) and definitions of delayed DTI intervals, categorized by both a binary threshold and a continuous measure in days. Sensitivity analyses were conducted as needed to explore heterogeneity across studies. Studies not amenable to meta -analysis were reported on in a narrative synthesis. We used Review Manager (RevMan 5.4, The Cochrane Collaboration, Oxford, United Kingdom) to perform our analyses. Publication bias was assessed with a funnel plot.
Publication biases
In our meta -analysis, several studies reported multiple effect sizes correlating with distinct patient cohorts, generally according to different time intervals. This multiplicity of effect sizes within the same studies introduced a potential redundancy when evaluating the presence of publication biases. To address this, each study was included only once in the funnel plot, irrespective of the number of effect sizes reported. We systematically selected the effect size associated with the earliest time interval group for each study. This selection criterion was applied to ensure consistency and avoid over-representation of any single study in our publication bias assessment.
Results
Literature Search & Baseline Characteristics
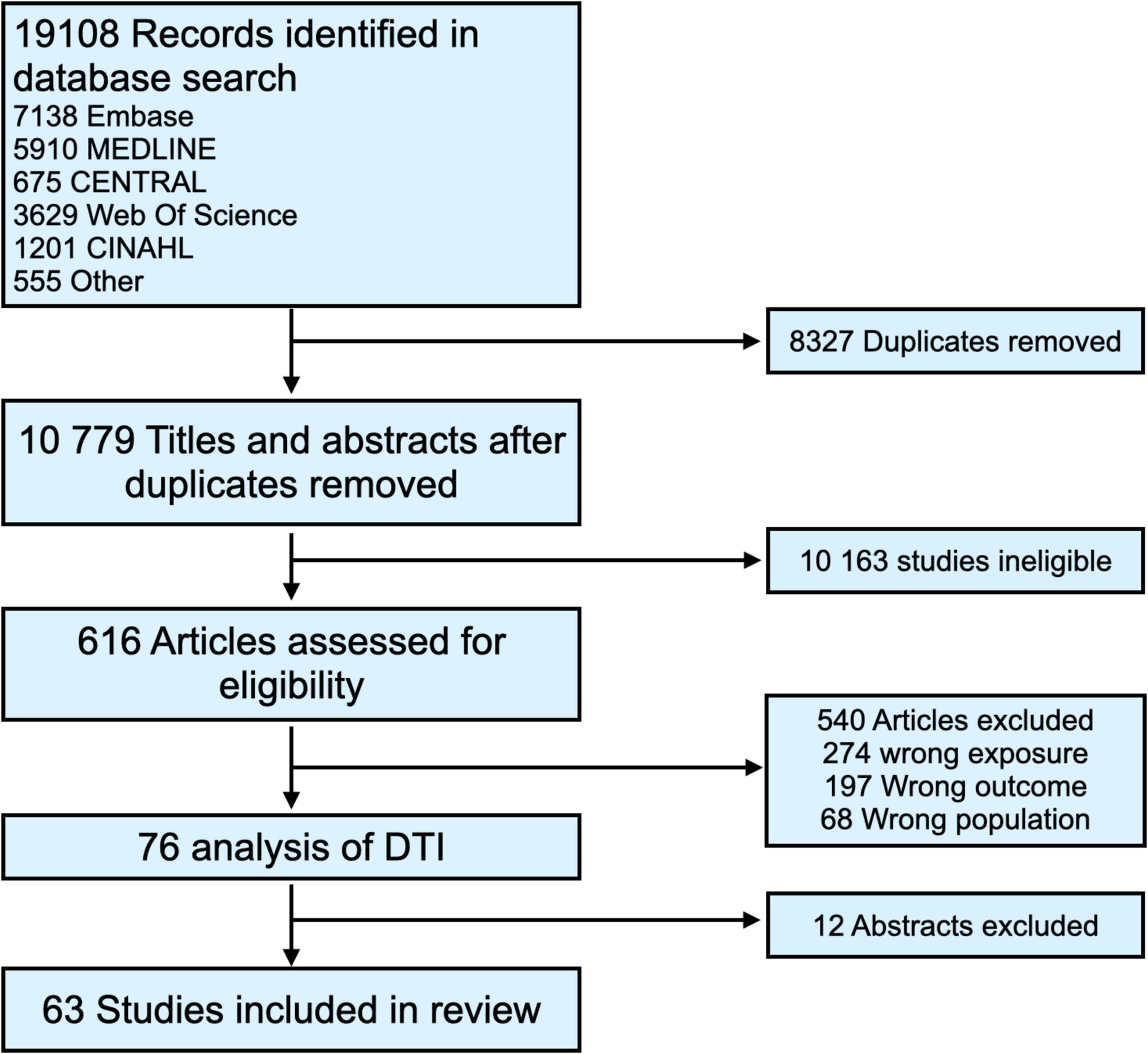
Our comprehensive search strategy initially identified 19,108 records. Post-duplicate removal, 10,779 titles and abstracts underwent screening, leading to 616 articles being assessed for full-text eligibility. Ultimately, 63 articles met our inclusion criteria, encompassing data from 873,718 patients ( Figure 1 ) . All included studies were real-world retrospective cohort studies. The majority were conducted in the United States, with 19 drawing data from the United States National Cancer Database (NCDB). The range of HNC diagnosis dates in these studies was broad, spanning from 1976 to 2020. Twenty-eight studies examined the impact of prolonged DTI across mixed sites , while others focused on specific sites: oropharynx (N = 11) , oral cavity (N = 7) , larynx and hypopharynx (N = 9) , nasopharynx (N = 7) , and sinonasal (N = 2) . The definition of delayed DTI varied, typically considered as over 30 days (± 4 days) but ranging from 17 to 100 days. Some studies also modeled DTI as a continuous variable. Definitions for delayed DTI intervals were primarily empiric, although some studies employed receiver operating characteristic (ROC) curves, recursive partition analysis, restricted cubic splines, and p-value distributions to identify optimal time thresholds. Detailed characteristics of the included studies are presented in Table S1 of the supplementary materials .
Overall survival
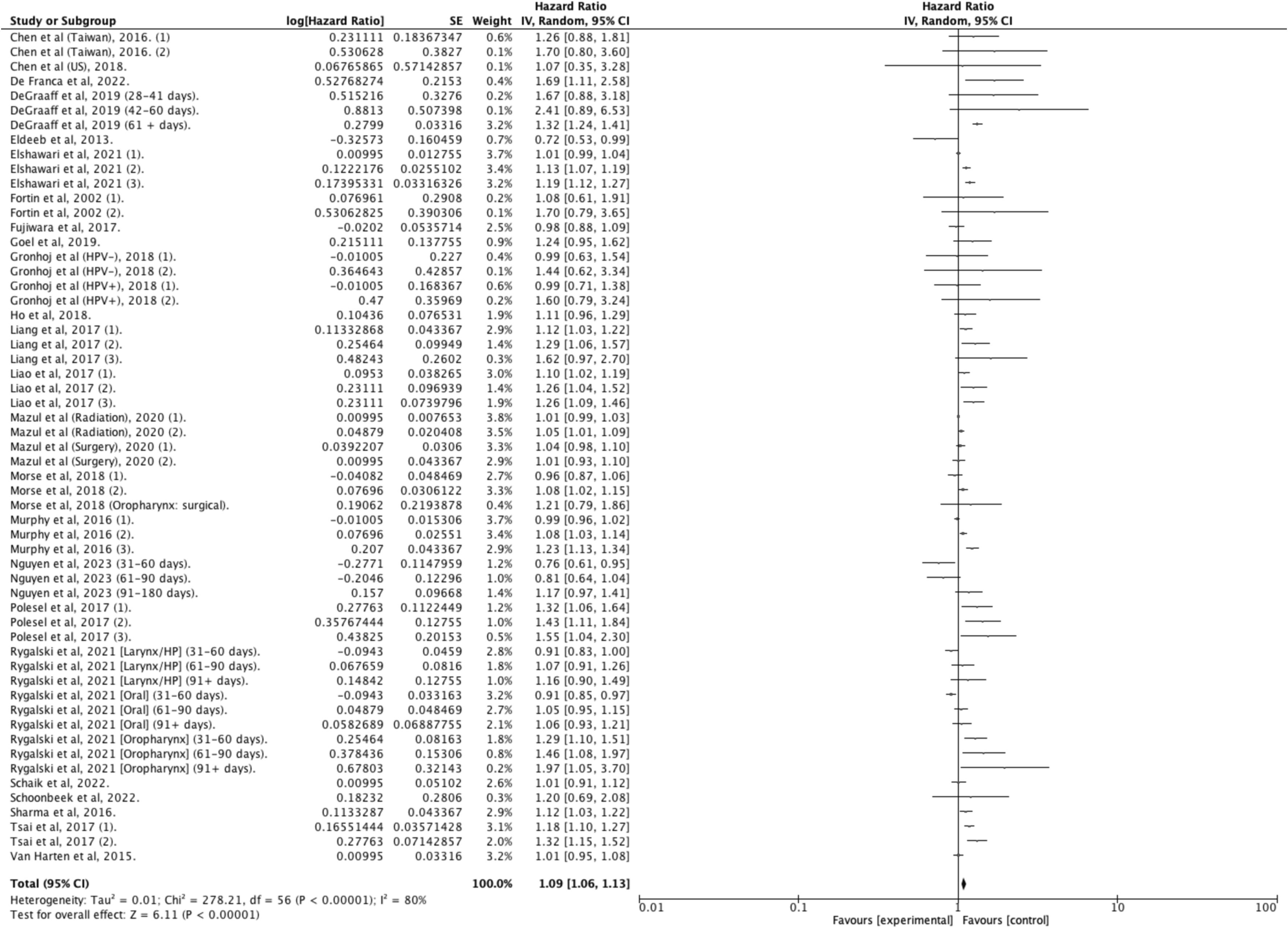
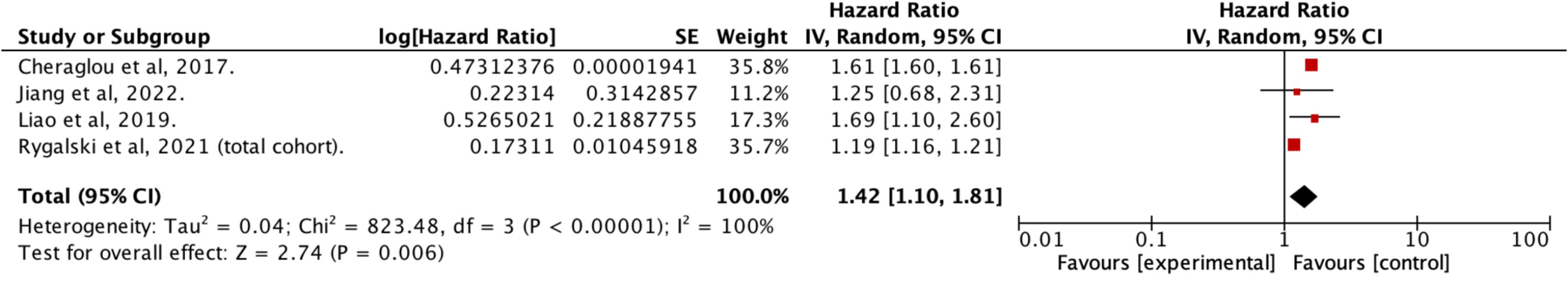
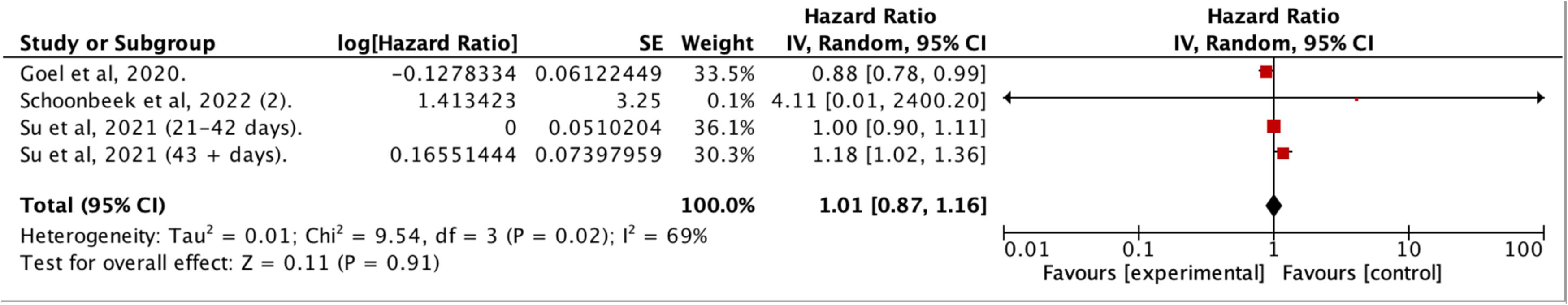
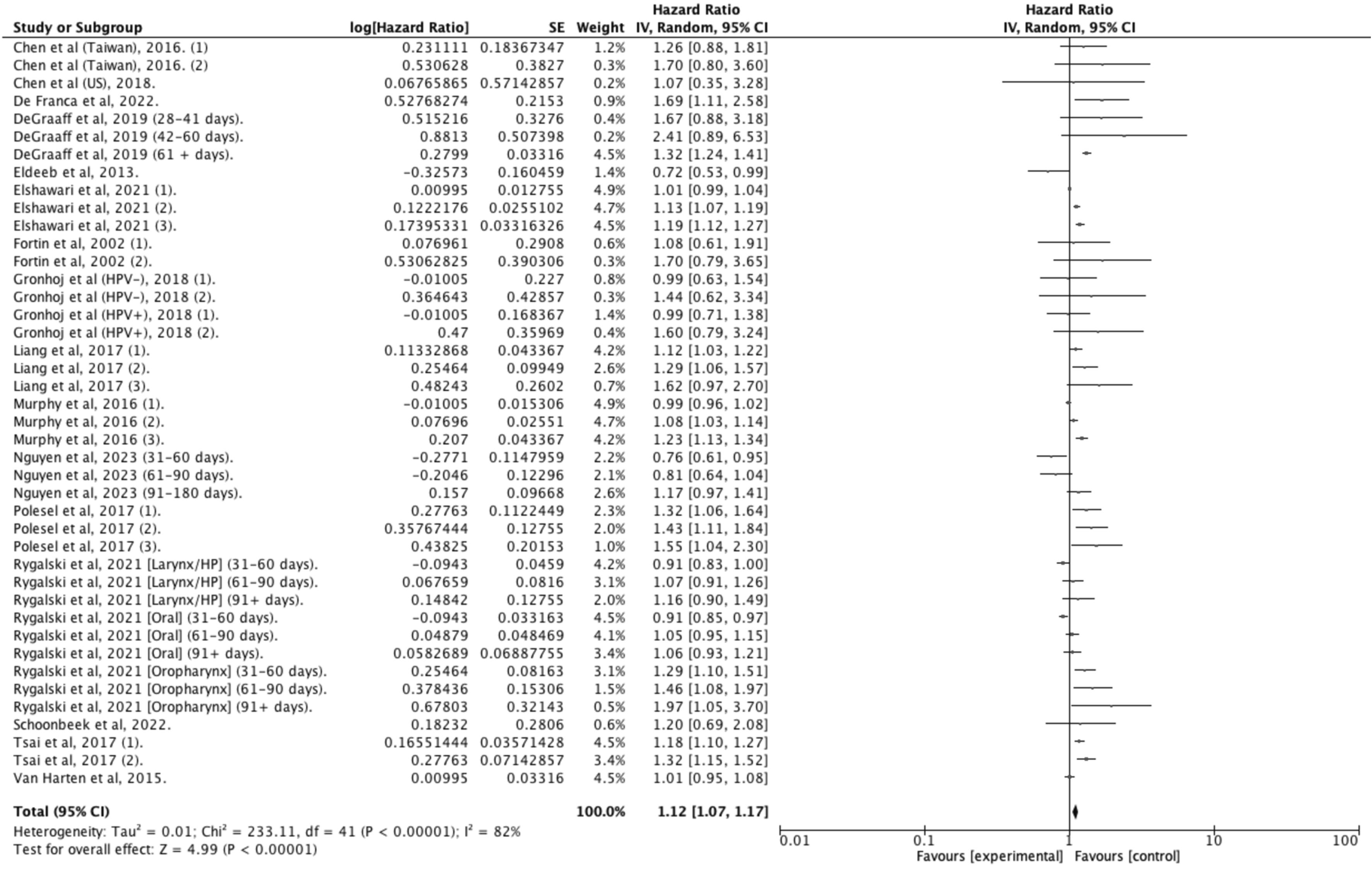
Fifty studies provided data on OS, with 33 reporting adjusted hazard ratios suitable for meta -analysis, but only studies sharing similar definitions of DTI were pooled together . In patients treated within 30 days of diagnosis, the pooled estimates indicated a 9 % improved survival for HNC compared to those with longer delays (aHR: 1.09 [1.06–1.13]; I 2 = 80 %; N = 466,220; 26 studies) ( Figure 2 ). This survival benefit was significant even with treatment initiation delayed beyond 60 days (aHR: 1.42 [1.10–1.81]; I 2 = 100 %; N = 44,659; 4 studies) ( Figure 3 ), but not for intervals of 25 days or less ( Figure 4 ) . A post-hoc sensitivity analysis, excluding studies with overlapping data sources (notably U.S. studies using NCDB data and Taiwanese studies using National cancer registry data), still showed a survival advantage for DTI shorter than 30 days (±3 days) (aHR: 1.12 [1.07–1.17]; I 2 = 80 %; N = 466,220; 17 studies) ( Figure 5 ). Subgroup analysis by anatomic site revealed a significant correlation between delayed DTI and overall survival in the oral cavity, oropharynx, and nasopharynx, but not in larynx & hypopharynx sites ( Figure S1-S4 respectively ). Subgroup analysis by treatment type revealed improved survival for surgical patients treated within 26 to 28 days and for non-surgical patients treated within 33 to 40 days ( Figures S5-S6 ). When DTI was modeled as a continuous variable, increasing each day, there was no significant association with overall survival (aHR: 1.00 [0.99 – 1.01]; I2 = 65 %; N = 5,661; 6 studies) ( Figure S7 ) . Seven studies were excluded from the pooled analyses due to survival outcome measures that could not be pooled, including unadjusted risk ratios, risk differences, or qualitative survival effect descriptions. Among these, five reported no significant correlation between delayed DTI and OS for thresholds ranging from 17 to 50 days . Two studies indicated a significantly better survival for treatments initiated within 20 and 21 days but did not quantify this association . Overall, the certainty of evidence for our primary outcome was deemed to be moderate ( Table 1 ).
| Outcome | № of Studies | Study Design | № of Patients | Effect Size (95 % CI) | Certainty | Importance |
|---|---|---|---|---|---|---|
| Overall Survival | 17 | Observational Studies | 466,220 | HR 1.12 (1.07 to 1.17) | ⊕⊕⊕○ Moderate | CRITICAL |
| Disease-Free Survival | 5 | Observational Studies | 13 479 | HR 1.13 (0.91 to 1.40) | ⊕○○○ Very Low | CRITICAL |
| Distant metastasis | 2 | Observational Studies | 958 | HR 1.81 (0.60 to 1.5.46) | ⊕○○○ Very Low | CRITICAL |
| Locoregional Recurrences | 6 | Observational Studies | 2805 | NA | ⊕○○○ Very Low | CRITICAL |
| Local Recurrences | 4 | Observational Studies | 1584 | NA | ⊕○○○ Very Low | CRITICAL |
| Health Related Quality of Life | 2 | Observational Studies | 289 | NA | ⊕⊕○○ Low | IMPORTANT |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree