Year
Authors
Contribution
1765
Morgagni JP [1]
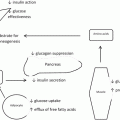
Macroscopic anatomic description of the association between visceral obesity and CV disease
1921
Hitzenberger K [2]
Description of the relationship between diabetes mellitus and hypertension
1922
Marañon G [3]
Description of the relationship diabetes mellitus and hypertension
1923
Kylin E [4]
Description of a syndrome of diabetes mellitus, hypertension, and hyperuricemia
1947
Vague JP [5]
Description of the android and gynoid types of obesity
1956
Vague JP [5]
Connection between android obesity and diabetes, hypertension, gout, and atherosclerosis
1966
Camus JP [10]
Description of the metabolic tri-syndrome: gout, diabetes, and hyperlipidemia
1967
Avogaro P [11]
Description of the plurimetabolic syndrome (hyperlipidemia, obesity, diabetes, hypertension)
1968
Menhert H, Kuhlman H [14]
Description of the syndrome of affluence
1977
Haller H [12]
Relationship between the metabolic syndrome and atherosclerosis
1980
Albrink MJ [8]
Relationship between obesity, hypertriglyceridemia, and low HDL-cholesterol
1981
Hanefeld M, Leonhardt W [13]
Metabolic syndrome: T2DM, hyperinsulinemia, obesity, hypertension, hyperlipidemia, gout, and trombophilia
1982
Kissebah AH [7]
Description of the disparate morphology and metabolic behavior of fat cells in different body fat distributions
1988
Reaven GM [17]
Syndrome X (insulin resistance as the common link)
1989
Kaplan NM [20]
The deadly quartet (central adiposity, IGT, hypertriglyceridemia, hypertension)
1991
De Fronzo RA, Ferrannini E [21]
Description of the multifaceted insulin resistance syndrome
1992
Larsson B [9]
Relationship between body fat and sex differences in cardiovascular disease
Terms similar to MetS were used to describe the association of cardiovascular risk factors already in the mid-1960s of last century. Camus named “metabolic tri-syndrome” the association of gout, diabetes, and hypelipidemia [10]. Avogaro proposed the term “plurimetabolic syndrome” to describe the association of obesity, diabetes, hyperlidemia, hypertension and CVD [11]. Finally, in 1977 Haller defined a “metabolic syndrome” as the confluence of obesity, diabetes mellitus, hyperlipoproteinemia, hyperuricemia, and hepatic esteatosis [12]. Of note, the authors underlined the potentiating effect of the combination of risk in atherosclerosis. The term “metabolic syndrome” was also used by Hanefeld and Leonhardt in 1981 to depict the association of diabetes, hyperinsulinemia, obesity, hypertension, hyperlidemia, gout, and thrombophilia [13]. From the early descriptions of the cluster of CVD risk factors, it was underscored that lifestyle was associated with the presence of the metabolic disturbances [3]. This was the basis for Mehnert and Kuhlmann to jointly name the clinical manifestations described above as “syndrome of affluence” [14].
On the occasion of the Banting Medal address at the 1988 annual meeting of the American Diabetes Association, Gerald M Reaven first introduced the term Syndrome X [15]. According to its pathophysiology, insulin-resistant nondiabetic individuals would be at increased risk to be somewhat glucose intolerant, hypertensive, and present with a form of dyslipidemia best described by the presence of elevated plasma triglycerides and low plasma HDL-cholesterol concentrations. The notion of a syndrome was proposed by Reaven to acknowledge that insulin resistance underlies several downstream manifestations. The number of these alterations present in a particular individual would vary depending upon the presence of additional factors. For instance, type 2 diabetes would not occur in the presence of insulin resistance unless a subject is unable to secrete enough insulin to overcome the defect in insulin action [16]. The X in the name of the entity was used to capture attention to the fact that the importance of insulin resistance as CVD risk factor was relatively unknown [17]. As a whole, the term Syndrome X served Reaven to emphasize the importance of insulin resistance and its manifestations as cardiovascular risk factors. The list of manifestations associated with insulin resistance was further expanded by several authors following the initial description by Reaven and will be discussed in the next chapter in this book. As insulin resistance was deemed the underpinning mechanism of Syndrome X, obesity or body fat distribution were not in the definition of the syndrome. The group by Reaven had shown that not all obese individuals are insulin resistant, and reciprocally nor are all insulin resistant individuals are obese [18]. As derived from the seminal study of the European Group for the Study of Insulin Resistance, only approximately 25 % of the variability in degree of insulin resistance in nondiabetic individuals would be accounted for by differences in degree of obesity [19].
It was only 1 year after Reaven’s Banting Medal address, that Kaplan vindicated again the importance of upper-body obesity as major contributor to other CVD risk factors often times present in some individuals [20]. Kaplan defined the deadly quartet as the combination of central adiposity, glucose intolerance, hypertriglyceridemia, and hypertension. The author acknowledged the importance of insulin resistance and the accompanying hyperinsulinemia as key intermediaries in the pathogenesis of the cluster of CVD risk factors. Furthermore, Kaplan recognized that genetic and environmental factors would be involved in its pathogenesis, since each of the different components of the quartet may occur in the absence of the others. However, following on Vague’s work, he call into attention the fact that obesity was being oversight as a major contributor to the CVD risk not captured by the traditional CVD risk factors. The inclusion of obesity in a multifaceted syndrome characterized by insulin resistance/hyperinsulinemia was further endorsed by DeFronzo and Ferrannini in 1991 [21].
In summary, this historical overview clearly underscores that the observations on the confluence of certain CVD risk factors in some individuals more often than by chance are not novel. Although some disagreement on its pathophysiology exists, historical concordance could be found in that such CVD risk factors are tightly linked to our lifestyle and would be alleviated by means of interventions aiming at lifestyle modification.
1.2 The Definition of the Metabolic Syndrome
In 1998, the World Health Organization (WHO) was first in proposing a formal definition of the MetS [22]. Several organizations followed (Table 1.2) [24–30]. Nonetheless, despite this sequence starting 15 years ago, the MetS definition still appears to be a work in progress and is seen rooted in controversy regarding its clinical utility [23].
Table 1.2
Components of the metabolic syndrome according to the definitions proposed by different organizations
WHO | EGIR | NCEP-ATP III | IDF | AHA/NHLB | IDF-AHA/NHLB |
|---|---|---|---|---|---|
Glucose intolerance and/or insulin resistance (hyperinsulinemic euglycemic clamp) | Elevated fasting insulinemia (upper 25th percentile of nondiabetics) | WC ≥102 cm in males, ≥88 in females | Elevated waist circumference (Population specific) | WC ≥102 cm in males, ≥88 in females | Elevated waist circumference (Population specific) |
Central obesity (males W/H > 0.9, females >0.85) or BMI > 30 kg/m2 | FPG ≥110 mg/dl, but not diabetic | FPG ≥110 mg/dl | FPG ≥100 mg/dl or drug therapy for ↑ glucose | FPG ≥100 mg/dl or drug therapy for ↑ glucose | FPG ≥100 mg/dl or drug therapy for ↑ glucose |
BP ≥160/90 mmHg | SBP ≥140 mmHg or DBP ≥90 mmHg or drug therapy for ↑ BP | SBP ≥130 mmHg or DBP ≥85 mmHg | SBP ≥130 mmHg or DBP ≥85 mmHg or drug therapy for ↑ BP | SBP ≥130 mmHg or DBP ≥85 mmHg or drug therapy for ↑ BP | SBP ≥130 mmHg or DBP ≥85 mmHg or drug therapy for ↑ BP |
Triglycerides ≥150 mg/dl and/or HDL-cholesterol <35 mg/dl in males or <39 mg/dl in females | Triglycerides ≥2.0 mmol/L and/or HDL < 1.0 mmol/L | Triglycerides ≥150 mg/dl | Triglycerides ≥150 mg/dl or drug therapy for ↑ triglycerides | Triglycerides ≥150 mg/dl or drug therapy for ↑ triglycerides | Triglycerides ≥150 mg/dl or drug therapy for ↑ triglycerides |
Microalbuminuria (UAER ≥20 μg/min or albumin/creatinine ≥20 mg/g) | WC ≥94 cm in males, ≥80 in females | HDL-cholesterol ≤40 mg/dl in males or ≤50 mg/dl in females | HDL-cholesterol ≤40 mg/dl in males or ≤50 mg/dl in females for ↓ glucose | HDL-cholesterol ≤40 mg/dl in males or ≤50 mg/dl in females for ↓ glucose | HDL-cholesterol ≤40 mg/dl in males or ≤50 mg/dl in females for ↓ glucose |
By providing a definition of the MetS, the group of WHO experts aimed at emphasizing the CVD risk associated with the coexistence in one individual of hypertension, upper body obesity, and dyslipidemia, with or without hyperglycemia [22]. They call attention to the fact that despite each component of the cluster conveying increased CVD risk, the combination of the different component was considered much more powerful. Furthermore, for those with normal glucose tolerance the concurrence of other components would put them at risk for future diabetes. The definition was presented embedded in a report on the diagnosis and classification of diabetes. For the diagnosis of the MetS either some degree of glucose intolerance and/or insulin resistance (as assessed from a hyperinsulinemic euglycemic clamp) was required. The diagnosis of the MetS would be made if two additional risk factors were present (Table 1.2).
Shortly after the WHO report had been published, the European Group for the Study of Insulin Resistance (EGIR) suggested some amendments to the proposed definition (Table 1.2) [24]. The EGIR experts considered as premises that (1) the diagnosis of the MetS was intended at easily identifying a group of patients with mild anomalies which, in combination, increase CVD risk, and (2) that insulin resistance was the underlying mechanism. From that perspective, the EGIR experts proposed fasting plasma insulin as simple measure of insulin sensitivity and to restrict the diagnosis of the MetS to nondiabetic individuals. In the presence of insulin resistance, the MetS would be diagnosed in the presence of two additional risk factors: impaired fasting but nondiabetic hyperglycemia, enlarged waist circumference, mildly elevated blood pressure, and elevated triglycerides and/or low HDL-cholesterol (Table 1.2). To strength the simplicity and feasibility of the diagnosis, the EGIR proposal left out of the definition the microalbuminuria criterion that was part of the WHO proposal.
In 2001, a new set of diagnostic criteria for the MetS was issued by the National Cholesterol Education Program (NCEP-ATPIII) (Table 1.2) [25]. The NCEP-ATPIII criteria aimed at easily identifying subjects in whom the CVD risk reduction associated with LDL-cholesterol lowering therapy could be threatened because of the presence of metabolic abnormalities often times associated with overweight or obesity. It was acknowledged that insulin resistance, a proinflammatory state, and a prothrombotic state were part of the syndrome but were left out of the definition because these were unfeasible to detect in routine clinical practice. At variance with previous definitions no single criterion was required for the diagnosis, but rather the diagnosis of the MetS was made in the presence of any combination of three of a set of five components. As previously endorsed by others, the MetS was considered a risk enhancer of CVD and T2DM. Nonetheless, as suggested by the WHO, the diagnosis of the MetS was applicable to subjects with T2DM.
The American Association of Clinical Endocrinologists (AACE) was next (2003) in joining the work in progress towards a definition of the MetS [26]. The criteria included were similar to those proposed by the WHO and ATP (obesity, elevated blood pressure, elevated triglycerides, low HDL-cholesterol), but the number of risk factors needed was not specified and the diagnosis was left to clinical judgment. As for the EGIR, the diagnosis would no longer apply when T2DM is present. Additional factors to inform clinical judgment included a family history of T2DM, hypertension or CVD, polycystic ovarian syndrome, advancing age, or a sedentary lifestyle.
In 2006, a new proposal came from the International Diabetes Federation (IDF) (Table 1.2) [27]. The proposal aimed at an easy to use and worldwide valid definition of a set of criteria that would allow the identification of subjects at considerably increased risk of developing CVD and/or T2DM. The IDF experts considered the obesity epidemic to be one of the main drivers of the high prevalence of the MetS. The 2001-ATP III definition was used as a starting point to be modified and updated to reflect current knowledge. At variance with the NCEP-ATPIII definition, the IDF proposal included central obesity as required criterion for the diagnosis. The rationale for this requirement was that central obesity was found more strongly correlated with the other MetS components than is any other parameter. Gender and ethnic-group specific cut-points for the waist circumference were proposed to acknowledge group-differences in body fat distribution. Two of for additional factors were required for the diagnosis of the MetS (Table 1.2). Of note, contemporarily to the IDF proposal, the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) slightly modified the NCEP-ATPIII criteria but did not obligatorily mandated an enlarged waist circumference for the diagnosis of MetS to be made [28]. The AHA/NHLBI considered that the different consideration of the obesity criterion between the two proposals would have minor effects since most individuals with the MetS according the AHA/NHLBI criteria would test positive for it.
The remaining differences between the IDF and the AHA/NHLBI were discussed in a joint meeting in 2009 and resulted in a harmonized definition of the MetS (Table 1.2) [29]. As a result, abdominal obesity was not considered any more a prerequisite for the diagnosis but rather given equal consideration to the other four components. The diagnosis would be made in the presence of any combination of at least three out of the five agreed criteria (Table 1.2). Interestingly, it was recommended that until more data was available the IDF cut-point for waist circumference were used in non-Europeans, and either the IDF (male ≥94, female ≥80) or AHA/NHLBI (male ≥102, female ≥88) for people of European origin. As previously proposed separately by the two organizations the diagnosis of the MetS would not exclude subjects with T2DM.
The final chapter of this evolutionary history was made public in 2010 and came, interestingly, from a WHO expert consultation [30]. In our opinion, that report contains several important statements. First, it was acknowledged that a formal diagnosis of the MetS is rarely made in routine clinical practice and has not been widely adopted in national guidelines for the prediction of CVD or T2DM. Second, the MetS should be considered as a pre-morbid condition rather than a clinical diagnosis. Thus, the diagnosis of the MetS does not apply to those with already existing T2DM or CVD. Third, efforts should be placed in elucidating the mechanisms underlying the clustering of the different components of the MetS rather than in developing new or revised definitions. Finally, it was concluded that despite the MetS could be useful as educational concept, it has limited practical utility as management tool.
In summary, over the last two decades we have witnessed a work in progress in search of clinically useful definition of the MetS. The existence of multiple definitions for the MetS has inevitably led to confusion. Proposals have varied depending on the underlying views of the proponents. It looks as if at the current stage we have not succeeded in our aim.
1.3 What Is in the Diagnosis?
According to the current of state of the art, the MetS could be best conceptualized as a recognizable cluster of physical and biochemical abnormalities occurring in one individual more often than expected by chance and for which the direct underlying cause is not well understood. The use of statistical analysis techniques appears to confirm the view that the different components of the MetS are part of a single entity [31]. In sharp contrast, over the last years the MetS has rather been used as clinical tool for the identification of individuals at high risk for CVD and T2DM. As a result, the concept of the MetS has been distorted and questioned [23, 32].
A recent systematic review and meta-analysis of 87 studies, in which either the NCEP-ATPIII or the AHA/NHLB definition was used, clearly illustrates the predictive value of the MetS for CVD outcomes [33]. The diagnosis of the MetS was associated with a twofold increase in risk for incident CVD, CVD mortality, myocardial infarction, and stroke. Risk estimates varied little whether derived from any of the two definitions. Moreover, the estimated relative risk for CVD mortality, myocardial infarction, and stroke were only slightly smaller when the analysis was restricted to the subgroup of nondiabetic subjects with the MetS. The estimated relative risks were, respectively, 1.75 (95 % CI: 1.19–2.58); 1.62 (95 % CI: 1.31–2.01); and 1.86 (95 % CI: 1.10–3.17). However, to what extent the diagnosis of the MetS accomplishes the goal of identifying individuals at high-risk for CVD is less obvious.
The performance of the MetS as compared to the widely used Framingham Risk Score (FRS) for the identification of subjects at risk for CVD has been reported in several studies including different populations [34–37]. In 2004, Stern et al. found the MetS had a lower sensitivity and was associated with a higher rate of false-positivity as compared to the FRS for the identification of incident CVD when applied to the San Antonio Heart Study population [34]. Likewise, McNeil et al. found the MetS did not improve coronary heart disease prediction beyond the level achieved by the FRS when applied the nondiabetic and CVD-free population participating in the Atherosclerosis Risk in Communities study [35]. Similar results were reported by Woodward [36] and Wannamethee [37] in prospective studies in which either Scottish men or women or British men with no history of CVD at baseline were followed up to 13.7 years and 20 years respectively. Although an in depth discussion of the reasons accounting for the poorer performance of the MetS as compared to the FRS in predicting CVD can be found elsewhere [30] and is beyond the scope of this review, we would like to emphasize three aspects. First, predicting tools such as the FRS may be more informative for risk estimation because of the inclusion of a set of unrelated factors rather than a set of intertwined components arising, potentially, from a single underlying mechanism. Second, whereas the FRS provides an estimate of absolute risk for CVD, the MetS informs on an individual’s relative risk compared to those without the MetS. Thus, the diagnosis of the MetS would not represent an equivalent risk for someone with an absolute risk in the low range as compared to someone with a high baseline risk. Finally, in a recent report involving Finnish and Swedish populations, no difference was found between the predictive value of CVD of a full definition of the MetS and its individual components [38]. Thus, discussion of the predictive value of the MetS in predicting CVD clearly exemplifies how the misuse of the MetS conceptual framework has resulted in distortion of its significance.
The ability of the MetS in predicting incident T2DM as compared to simpler measures has also been questioned [23]. It has been shown that the relative risk for incident T2DM is up to five times higher in individuals with the MetS compared with those without the syndrome [39]. Despite some controversy exists, it has been suggested that fasting plasma glucose accounts for a large proportion of the T2DM predictive capacity of the MS [40, 41]. Furthermore, Stern et al. demonstrated that the Diabetes Predicting Model outperformed the MetS in predicting T2DM in the San Antonio Heart Study and Mexico City Diabetes Study [34]. Numerous diabetes risk scores are now available that provide good estimates of the chance of individuals developing diabetes in the mid- or long-term future [42]. For that matter, the MetS does not appear to be the universal ideal diabetes risk score.
To put what is in the diagnosis of the MetS into clinical perspective, we may wonder if being diagnosed with the MetS because of the presence of fasting plasma glucose of 101 mg/dl, systolic blood pressure of 137 mmHg, and fasting triglycerides of 151 mg/dl, would imply a fivefold larger risk for CVD and a twofold larger risk for T2DM as compared with the same individual not fulfilling any of the MetS components because of a fasting plasma glucose of 99 mg/dl, systolic blood pressure of 134 mmHg, and fasting triglycerides of 149 mg/dl. In brief, we could well conclude that the MetS is not the best available tool for the prediction of CVD or T2DM. Further than this being a scientifically or clinically unresolved question, the low degree of implementation of the MetS as screening tool in CVD or T2DM prevention programs throughout the world would support this view [30]. Nonetheless, this does not detract from the fact that the MetS enfolds the concept of a number of cardiovascular risk factors presenting together in some individuals, and that these risk factors either individually or as group may benefit from lifestyle interventions.
1.4 Current Management Caveats
Major limitations for advancing in the definition of the best management strategies for the MetS include the lack of a consensus definition, the vagueness of its predictive ability for CVD and T2DM outcomes, and the lack of a unifying pathogenic hypothesis (Table 1.3). In the absence of an agreed definition, it is hard to set the proper patient selection criteria to be used in clinical trials evaluating therapeutic strategies and it becomes delicate to compare results across different studies using different definitions. Variation in the strength of association between the 16 potential combinations leading to the diagnosis of the MetS and CVD and/or T2DM hampers accurate trial design. The lack of a unifying underpinning mechanism questions whether the MetS is best treated targeting a single factor or, alternatively, confronting each component separately. At the end of the day, from the management point of view it looks as if we have not gone too far from the historical concept of the “syndrome of affluence” mentioned earlier in this chapter [3, 14]. Lifestyle modification aiming at weight loss and increased physical activity is the foundation of current management of the MetS. Although evidence coming from studies targeting specifically MetS patients is lacking, several studies have shown such lifestyle interventions simultaneously impact more than one component of the MetS [43]. However, it should be emphasized that further than adequate lifestyle modification, targeting each individual component is central to additionally decrease the CVD burden associated with the MetS.
Table 1.3




Major limitations in the management strategies of the metabolic syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree