Clinical criteria
1. Vascular thrombosis
One or more clinical episodes of arterial, venous, or small vessel thrombosis, in any tissue or organ. Thrombosis must be confirmed by objective validated criteria (i.e., unequivocal findings of appropriate imaging studies or histopathology). For histopathologic confirmation, thrombosis should be present without significant evidence of inflammation in the vessel wall.
2. Pregnancy morbidity:
(a) One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation
(b) One or more premature births of a morphologically normal neonate before the 34th week of gestation because of eclampsia, severe preeclampsia, or recognized features of placental insufficiency
(c) Three or more unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded
Laboratory criteria
1. Lupus anticoagulant present in plasma, on two or more occasions at least 12 week apart, detected according to the guidelines of the International Society on Thrombosis and Hemostasis
2. Anticardiolipin antibody of IgG and/or IgM isotype in serum or plasma, present in medium or high titer (i.e., ≥40 GPL or MPL, or greater than the 99th percentile), on two or more occasions, at least 12 weeks apart, measured by a standardized ELISA
3. Anti-β2-glycoprotein I antibody of IgG and/or IgM isotype in serum or plasma (in titer greater than the 99th percentile) present on two or more occasions, at least 12 weeks apart, measured by a standardized enzyme-linked immunosorbent assay
Definite APS is present if at least one of the clinical criteria and one of the laboratory criteria are met
Antiphospholipid Syndrome Based on Revised Sapporo Classification Criteria
The purpose of the Revised Sapporo APS Classification Criteria is to include homogeneous groups of patients in research. The revised version [4], compared to the original [1], includes aβ2GPI test as one of the laboratory criteria, 12 weeks instead of 6 weeks interval between two aPL tests, maximum of five years interval between the first aPL and clinical manifestations, and clarified definitions of laboratory thresholds.
While developing the original and the revised criteria, several studies were assessed for the causal role of aPL for clinical manifestations in experimental and clinical studies and the frequency of clinical manifestations in aPL-positive patients. The causal role of aPL was demonstrated in vivo for some (but not all) clinical manifestations [5, 6]. Although several clinical manifestations were frequent in aPL-positive patients [7, 8], suggesting their inclusion as classification criteria, only thrombosis and pregnancy morbidity were eventually selected. Non-criteria manifestations, for instance, livedo, valve disease, and aPL nephropathy, are not included in the criteria because of the lack of recognized causal relationship or statistical association with aPL and also to avoid heterogeneity. Detailed discussion of the limitations of the Revised Sapporo APS Classification Criteria [4] and an international effort to develop new evidence-based APS classification criteria [9] are discussed in Chap. 15.
Clinically Significant Antiphospholipid Antibody Profile
Not every positive aPL test is clinically significant. For instance, transient low titer aPL positivity is common during infections. In fact, more than two-thirds of aPL/aβ2GPI detected during infections are transient and not associated with clinical consequences [10]; aCL is the most frequent aPL during infections [10]. Thus confirmation of aPL on two occasions at least 12 weeks apart [4] is an important criterion. An isolated aCL/aβ2GPI test, especially low levels, should trigger an investigation to rule out infection.
The following points are important while correlating aPL tests with clinical events: LA correlates better than do aCL/aβ2GPI tests [11–14]; moderate to high titer (≥40 U or ≥99th percentile) aCL or aβ2GPI IgG/IgM (≥99th percentile) is better than are lower titers [15]; IgG isotype is better than IgM isotype [15]; and triple aPL (LA, aCL, and aβ2GPI) positivity is better than single or double aPL [16, 17]. Clinical judgment is required while interpreting aPL tests when the LA test is performed on anticoagulated patients, especially direct oral anticoagulants, which induce false positive results [18]; aCL or aβ2GPI IgG/IgM titers are in the lower range (20-40 U); only one aPL determination is available; and/or aCL or aβ2GPI IgA is the only positive ELISA test.
Antiphospholipid antibody tests that are not part of the current classification criteria, for instance, antiphosphatidylserine-prothrombin or anti-domain I β2GPI antibodies, may be more specific for APS diagnosis than are “criteria” aPL tests [19]. However, their use in clinical practice is limited due to the lack of standardization (discussed in a different chapter) and limited availability.
Clinical Heterogeneity of Antiphospholipid Antibody-Positive Patients
Antiphospholipid antibodies can result in a broad spectrum of manifestations: criteria APS – thrombotic, obstetrical, or both (Table 7.1), asymptomatic aPL (no thrombosis or pregnancy morbidity); non-criteria manifestations with/without APS classification; and CAPS (multiple-organ thromboses commonly associated with thrombotic microangiopathy).
Several systematic reviews and meta-analyses have been performed on non-criteria manifestations [20, 21]. A recent report summarized recommendations of the APS Clinical Features Task Force of the 14th International Congress on aPL (Rio De Janeiro, Brazil, September 2013); the task force concluded that thrombocytopenia, heart valve disease, renal microangiopathy (aPL nephropathy), chorea, and longitudinal myelitis should be included as part of a future APS Classification Criteria [22]. Table 7.2 summarizes recent meta-analyses assessing selected non-criteria aPL manifestations [20].
Table 7.2
Selected meta-analysis demonstrating the increased risk of clinical manifestations in antiphospholipid antibody (aPL)-positive systemic lupus erythematosus (SLE) patients compared to aPL-negative SLE patients [20]
Manifestations | Increased risk [OR (95% CI)] | |||
|---|---|---|---|---|
LA | aCL | aβ2GPI | “aPL” | |
Valve disease | 5.8 (2.9–11.8) | 5.6 (3.5–8.9) | N/A | 3.1 (2.3–4.2) |
Pulmonary hypertension | 2.0 (1.3–2.9) | 2.6 (1.3–5.4) | NS | 2.3 (1.7–3.2) |
Livedo reticularis | 5.7 (3.3–10.1) | 3.3 (2.0–5.3) | 4.7 (2.4–9.3) | 3.6 (2.4–5.4) |
Thrombocytopenia | 3.9 (2.8–5.4) | 1.9 (1.5–2.3) | 1.9 (1.0–3.8) | 2.5 (2.1–2.9) |
Hemolytic anemia | 3.7 (2.3–5.9) | 2.3 (1.7–3.1) | 2.6 (1.2–5.7) | 3.0 (2.2–4.2) |
Renal impairmenta | 5.3 (2.6–10.9) | 4.9 (2.0–12.1) | NS | 3.0 (2.0–4.6) |
“Non-criteria” are not necessarily independent from clinical criteria (Table 7.3). That is, heart valve disease is associated with cerebrovascular events [23], and livedo racemosa predicts stroke [24]. The mechanisms of non-criteria manifestations, for example, endothelial cell activation and vascular wall proliferation, are potentially relevant for criteria manifestations [25, 26]. Antiphospholipid-related nephropathy lesions can be either acute or chronic; acute lesions correspond with microthrombosis (criteria manifestation) [4], whereas chronic lesions correspond with microvascular wall abnormalities such as focal atrophy and intimal hyperplasia (non-criteria manifestation) [27].
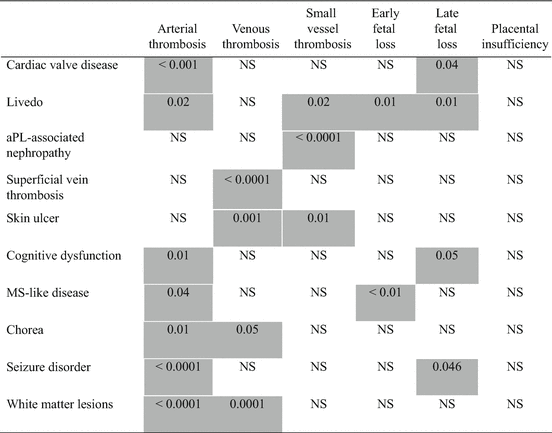
Table 7.3
Statistical associations (p value) between antiphospholipid syndrome (APS) clinical criteria and non-criteria manifestations among 600+ patients based on the analysis of the APS Alliance for Clinical Trials and International Networking (ACTION) Registry

Clusters of Antiphospholipid Antibody-Positive Patients
Cluster analysis (CA) is a data-driven method that groups patients so that patients in the same group (cluster) are more similar to each other than to those in other groups. Several studies have used CA to identify different clinical phenotypes in lupus [28], Parkinson’s disease, asthma, and inflammatory bowel disease. In APS, CA can characterize the broad spectrum of APS manifestations and to help us understand why patients have heterogeneous presentations.
Based on the APS Alliance for Clinical Trials and International Networking (APS ACTION ) clinical database analysis of 497 patients [29], three clusters with different combinations of clinical and laboratory features were identified (Fig. 7.1, Table 7.4):
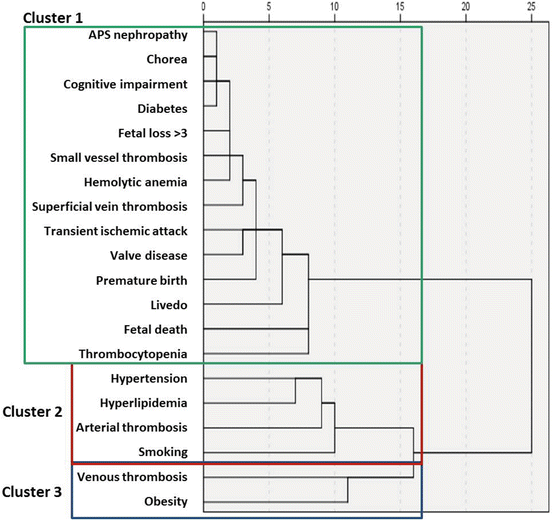
Cluster 1 – patients with no other autoimmune diseases, but with venous thromboembolism and triple-aPL positivity
Cluster 2 – patients with lupus, venous thromboembolism, aPL-related nephropathy, thrombocytopenia, hemolytic anemia, and positive LA test
Cluster 3 – older patients with arterial thrombosis, heart valve disease, livedo, skin ulcer, neurological manifestations, and cardiovascular (CVD) risk factors

Fig. 7.1
Clustering of the antiphospholipid antibody manifestations based on the analysis of the APS Alliance for Clinical Trials and International Networking (ACTION) Registry. In this analysis, hierarchical clustering was used which encompasses three steps: (a) calculating the distance (Euclidean) between means of patients/manifestations aiming to minimize the within-cluster variance; for instance, the distance between two patients with both arterial thrombosis and arterial hypertension is very low (similar patients); however, the distance between a patient with venous thrombosis/obesity and a patient with hemolytic anemia is high (no similarity); (b) linking the clusters in a dendrogram (the distance between these clusters is computed using the Euclidean distance, and the two nearest clusters are merged together to form a new cluster that replaces the two previous clusters; merging of the two nearest clusters is repeated until only one cluster is left; the tree diagram generated to illustrate the arrangement of the clusters produced by the clustering is termed a dendrogram); (c) comparing clusters of patients
Table 7.4
Cluster characteristics of antiphospholipid antibody-positive patients based on the analysis of the APS Alliance for Clinical Trials and International Networking (ACTION) Registry [29]
Variables, n (%) | Cluster 1 | Cluster 2 | Cluster 3 | p value |
|---|---|---|---|---|
Demographics | ||||
Total n | 179 | 180 | 138 | |
Mean age, year ± SD | 41.9 ± 11.6 | 42.3 ± 12.5 | 51.0 ± 12.4 a,b | <0.001 |
Female | 145 (81.0) c | 145 (80.6) c | 92 (66.7) | 0.004 |
Past medical history | ||||
Clinical criteria | ||||
Arterial thrombosis | 28 (15.6) | 51 (28.3)a | 95 (68.8) a,b | <0.001 |
Venous thrombosis | 84 (46.9) c | 85 (47.2) c | 45 (32.6) | 0.014 |
Small vessel thrombosis | 9 (5.0) | 11 (6.1) | 10 (7.2) | 0.712 |
Pregnancy morbidity | 73 (40.8) | 67 (37.2) | 42 (30.4) | 0.195 |
Non-criteria manifestations | ||||
Heart valve disease | 9 (5.0) | 6 (3.3) | 23 (16.7) a,b | <0.001 |
Livedo | 15 (8.4) | 26 (14.4) | 30 (21.7) a | 0.003 |
Skin ulcer | 6 (3.4) | 11 (6.1) | 14 (10.1) a | 0.046 |
Neurological manifestations | 22 (12.3) | 26 (14.4) | 58 (42.0) a,b | <0.001 |
aPL nephropathy | 2 (1.1) | 10 (5.6) c | 0 (0) | 0.002 |
Thrombocytopenia | 22 (12.3) | 45 (25.0) a | 22 (15.9) | 0.006 |
Other autoimmune diseases | ||||
None | 114 (63.7) b | 86 (47.8) | 79 (57.2) | 0.009 |
Systemic lupus Erythematosus | 25 (14.0) | 74 (41.1) a,c | 26 (18.8) | <0.001 |
Cardiovascular risk factors | ||||
Hypertension on medication | 14 (7.8) | 33 (18.3)a | 99 (71.7) a,b | <0.001 |
Diabetes on medication | 4 (2.2) | 5 (2.8) | 12 (8.7) a | 0.009 |
Hyperlipidemia on medication | 12 (6.7) | 31 (17.2)a | 65 (47.1) a,b | <0.001 |
Obesity | 31 (17.3) | 49 (27.2) | 60 (43.5) a,b | <0.001 |
Smoking | 44 (24.6) | 61 (33.9) | 74 (53.6) a,b | <0.001 |
Laboratory parameters | ||||
Antiphospholipid antibodies | ||||
Lupus anticoagulant | 129 (72.1) | 152 (84.4) a | 105 (76.1) | 0.017 |
Anticardiolipin antibodies | 166 (92.7) b,c | 63 (35.0) | 115 (83.3)b | <0.001 |
Anti-β2-glycoprotein I antibodies | 138 (77.1) b,c | 25 (13.9) | 73 (52.9)b | <0.001 |
Triple positivity | 99 (55.3) b,c | 13 (7.2) | 56 (40.6)b | <0.001 |
Other laboratory parameters | ||||
Hemolytic anemia | 2 (1.1) | 18 (10.0) a | 6 (4.3) | 0.001 |
Antinuclear antibodies | 104 (58.4) | 117 (65.7) c | 72 (52.2) | 0.05 |
dsDNA antibodies | 43 (24.0) | 61 (33.9) c | 23 (16.7) | 0.002 |
Low C3 | 20 (29.9) | 39 (49.4) a | 18 (48.6) | 0.039 |
A follow-up analysis of 290 patients with pregnancy history [30] identified four clusters:
Cluster 1 – older patients with arterial thrombosis and CVD risk factors
Cluster 2 – patients with pregnancy morbidity only
Cluster 3 – asymptomatic aPL-positive patients with aCL/aB2GPI
Cluster 4 – patients with venous thrombosis obesity, SLE, and LA
These analyses identify clinical phenotypes and suggest that these phenotypes are driven by different triggers or underlying diseases.
Antiphospholipid Antibody (as a Risk Factor) Versus Antiphospholipid Syndrome (as a Disease)
Independent of APS classification criteria, one way of grouping aPL-positive patients is:
Group 1: aPL is a bystander with no causative role in the clinical problem(s), for instance, a low titer aCL or aβ2GPI in the setting of an infection.
Group 2: aPL is a risk factor that potentially contributes to the clinical problem(s), for instance, deep vein thrombosis in an aPL-positive patients who is undergoing major surgery.
Group 3: aPL is the cause of the clinical problems, for instance, non-criteria manifestations of aPL.
Thus, during risk stratification and management decisions, it is important to categorize aPL-positive patients as much as possible, even if at times no clear-cut distinction exists between these groups.
Obstetric Antiphospholipid Syndrome
Recurrent miscarriages, fetal loss, placental insufficiency, or preeclampsia are the criteria manifestations of obstetric APS. They can occur in patients with or without prior thrombosis. The association of pregnancy loss and thrombosis has been described since the first reports of the syndrome [31].
Pregnancy Morbidity Included in the Antiphospholipid Syndrome Classification Criteria [32]
- (a)
One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus.
According to international consensus, fetal death is the most specific criterion for obstetric APS, confirmed by a systematic review [32] and two recent multicenter studies [32, 33]. High titer aCL and aβ2GPI are associated with a three- to fivefold increased odds of stillbirth [33], while LA positivity is the primary predictor of adverse pregnancy outcome after 12 weeks [34]. Approximately 10–15% of all clinically recognized pregnancies (independent of aPL) result in early miscarriage, most before eight weeks, after which the risk of pregnancy loss decreases significantly (3%). Chromosomal abnormalities, the main cause of spontaneous abortion in the general population, usually result in miscarriage at earlier gestational ages; the incidence decreases steeply after the first trimester, as other causes of fetal loss, such as APS, proportionally increase [35]. Congenital abnormalities, regardless of karyotype, play a significant role in all fetal loss. Obstetric APS diagnosis and classification exclude these alternative causes of fetal loss.
- (b)
One or more premature births of a morphologically normal neonate before the 34th week of gestation due to (i) eclampsia or severe preeclampsia defined according to standard definitions or (ii) recognized features of placental insufficiency.
Preeclampsia is relatively common in a general population (5–10% of all pregnancies) [36]. In APS patients, the incidence of preeclampsia is higher; half of the cases are classified as severe. The real frequency of aPL in patients with preeclampsia is still unknown, but many studies report a significant association between aPL and early-onset severe preeclampsia [37]. Recently, a meta-analysis of 12 studies and 8475 SLE patients showed an odds ratio of 2.86 (95% confidence interval [CI]: 1.37–5.98) for preeclampsia of any severity in SLE patients with aPL, compared to those without aPL [38].
Preeclampsia has a standard definition: systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg on two occasions, at least four hours apart at 20 or more weeks, and ≥300 mg protein/24-h urine collection in a previously normotensive patient. Severe preeclampsia is when the systolic blood pressure is ≥160 mmHg or diastolic ≥110 mmHg on two occasions, at least four hours apart, and/or HELLP syndrome (see below), renal insufficiency, pulmonary edema, and new-onset cerebral or visual disturbances such as headache or blurred vision occur. The occurrence of generalized tonic-clonic seizures defines eclampsia [39].
Intrauterine growth restriction due to placental insufficiency in APS patients is high (15–40%), although the definition of placental insufficiency is debatable [40, 41]. The Revised APS Classification Criteria describe placental insufficiency as: abnormal or non-reassuring fetal surveillance test(s), for instance, a nonreactive non-stress test, suggestive of fetal hypoxemia; abnormal Doppler flow velocimetry waveform analysis suggestive of fetal hypoxemia, for instance, absent end-diastolic flow in the umbilical artery; oligohydramnios, for instance, an amniotic fluid index of five centimeters or less; and a postnatal birth weight less than the 10th percentile for the gestational age [4]. The differential diagnosis of placental insufficiency includes SLE, multiple gestation, substance abuse (tobacco, alcohol, or cocaine), infections, genetic and structural disorders, and placental abnormalities [40].
To enhance the specificity of this criterion, the Revised APS Classification Criteria include only cases that are complicated by preterm delivery before 34 weeks. This criterion may be insensitive and nonspecific. Delivery is usually due to the intervention for fetal or maternal reasons; spontaneous preterm labor of an otherwise uncomplicated pregnancy would be unusual in APS [37].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree