© Springer Science+Business Media New York 2014
Fred Saad and Mario A. Eisenberger (eds.)Management of Castration Resistant Prostate CancerCurrent Clinical Urology10.1007/978-1-4939-1176-9_1414. Defining Clinical Endpoints in Castration-Resistant Prostate Cancer
(1)
Department of Solid Tumor Oncology, Cleveland Clinic Taussig Cancer Institute, 9500 Euclid Avenue/R35, Cleveland, OH 44195, USA
(2)
Department of Urology, Cleveland Clinic Glickman Urological and Kidney Institute, 9500 Euclid Avenue/R35, Cleveland, OH 44195, USA
Keywords
Castration-resistant Prostate Cancer (CRPC)endpointsprostate-specific antigen (PSA)progression-free survival (PFS)overall survival (OS)overall response rate (ORR)quality of life (QOL)Introduction
Although this is an exciting time for prostate cancer (PCa) therapeutics, drug development in men with castration-resistant prostate cancer (CRPC) faces significant challenges. Disease biology and the well-recognized heterogeneity of prostate cancer are some of the inherent disease-specific issues that continue to affect trial design [1]. Similarly, traditional endpoints used in early phase I and II studies do not appear to be useful when evaluating survival outcome in phase III trials. Adding complexity to a challenging area, some newer PCa trials design guidelines do not align with current US Food and Drug Administration (FDA) requirements necessary for drug registration and approval [2]. To date, there are no validated surrogate endpoints capable of predicting benefit of early treatment in men with metastatic castration-resistant prostate cancer (mCRPC). Historical endpoints such as prostate-specific antigen (PSA), Response Evaluation Criteria in Solid Tumors (RECIST), and progression-free survival (PFS) have failed to reliably predict survival benefit in this patient population [3]. Although clinically relevant, assessments of QOL and pain have not been reliable trial endpoints. Additionally, changes in the ubiquitous biomarker PSA, an androgen receptor (AR) gene product may be relatively uninformative when used in the setting of non-AR directed therapy and in general has not been found to correlate with more accepted outcome measures such as survival [4].
The evolving complexities of the design and conduct of a clinical trials, FDA requirements for registration, and the routine clinical use of a variety of endpoints are now increasingly challenged by the rapidly evolving understanding of the biology of CRPC and the importance of the AR as an therapeutic target for drug development [5, 6]. Adding to the challenge is the widespread availability of the AR targeting agents enzalutamide and abiraterone acetate (AA) without a prospectively tested and validated understanding of their optimal use in terms of sequence and patient selection [7–10].
Another pressing question is how to incorporate non-AR targeting therapeutics such as immunotherapy into routine clinical practice as these agents by virtue of their mechanism of action may require “different” endpoints, especially if they are used early in the disease course. Sipuleucel-T is a perfect example of the apparent disconnect between traditional endpoints such as prostate-specific antigen (PSA) response and objective response rate (ORR) and overall survival [11].
Defining rational and practical endpoints that can be used in CRPC trial design is desperately needed, especially as clinical oncology is rapidly, albeit uncertainly, moving towards an era of genomics and precision medicine while at the same time shifting rapidly to an era of cost-containment making new drug development that much more challenging. In this chapter we review traditional endpoints that have historically been used in CRPC trial design. We also discuss potential new strategies that could be incorporated in future CRPC studies.
Understanding Castration-Resistant Prostate Cancer (CRPC)
Although from the perspective of managing an individual patient, CRPC remains a very heterogeneous disease, advances in the biology of CRPC have codified the importance of AR pathway and its role in the pathogenesis and progression to a castration-resistant state [5, 12]. Among the challenges when attempting to define outcomes in men with prostate cancer has been the lack of validated surrogate markers that can be used to predict overall survival. Disease heterogeneity also plays a role in the evolution to castration-resistant disease given the likelihood that multiple pathways in AR resistance are involved and with the clinical definition of castration-resistant disease somewhat empirically defined as disease progression be it biochemical, i.e. PSA-only or with overt radiographic and/or symptomatic progression in the setting of a “castrate level” (<50 ng/dl) of testosterone [12]. As such some men can present with serologic progression-only while others develop symptomatic disease. Similarly, many others are detected by “routine” imaging studies. More recently the importance of disease related symptoms when selecting CRPC patients for clinical trials has been increasingly considered and included into study designs. Differences among these groups of patients, i.e. asymptomatic versus minimally symptomatic versus symptomatic, have become increasingly relevant when interpreting trial results as more contemporary studies have included a placebo component and utilize pain and quality of life (QOL) instruments in their trial design [13, 14]. With the availability of Sipuleucel-T, abiraterone acetate (AA) and enzalutamide, the number of previous therapies, treatment sequence, and the possibility of early introduction of chemotherapy will likely impact the selection of endpoints for future clinical trials.
Additional challenges include the incorporation of serum and tissue biomarkers in mCRPC trial designs. In this evolving era of precision medicine where we are faced with understanding the role and the potential utility of next generation genomics, well-defined strategies that integrate biologically relevant markers will be needed to facilitate progress towards a more rational approach to the management of men with CRPC [15, 16].
Traditional Endpoints in CRPC Trials
PSA Response
Since its introduction in the late 1980s PSA has been the most commonly used biomarker to both evaluating treatment efficacy and predicting prognosis for patients with advanced PCa [17, 18]. While undetectable PSA values are often achieved shortly after primary, curative intent therapy with either surgery or radiotherapy, absolute PSA values, percentage of PSA decline, and other methods of assessing PSA kinetics are more difficult to interpret in the advance disease setting [19, 20]. Assay variability and the capriciousness of PSA assessments outside the context of trial requirements have complicated the interpretation of clinical studies as many physicians rely on PSA values when making treatment decisions in their routine clinical practice. This impact may be felt to a greater extent when management changes are considered for men based on PSA progression when receiving therapeutic agents with non-AR-dependent mechanisms of action. The relationship between objective response and PSA changes over time is also challenged by the common disconnect between PSA decline and tumor burden reduction often observed in men receiving therapy for mCRPC. Historically PSA response and time to PSA progression have been incorporated in prostate cancer clinical trials [21]. Retrospective studies conducted in the late 1980s suggested that PSA declines could be interpreted as a good indicator of treatment response [22, 23]. Investigators at the University of Michigan provided preliminary evidence that men who failed to achieve a PSA decline >50 % while receiving chemotherapy doubled their risk of death compared to those able to achieve that level of biochemical response [23]. Another example of the challenges of using PSA response as an endpoint was illustrated by a translational study of suramin, a highly charged polysulfonated naphthylurea capable of binding a number of proteins, including a variety of growth factors such as basic fibroblast growth factor. Suramin inhibited PSA secretion with no cytotoxic effect and there was no association between the percentage of PSA decline (<50 versus ≥50–75 % versus ≥75 %) and survival [24]. The results of this analysis conflicted with the results of their phase III study that evaluated Suramin versus hydrocortisone in men with mCRPC where a post-treatment PSA decline of ≥50 % lasting ≥4 weeks was associated with longer PSA and overall survival. This difference was maintained after adjusting for burden of disease and baseline PSA value [25].
The uncertain utility of PSA as a response parameter and inability to define standard endpoints for trials in advanced prostate cancer led a number of academic investigators involved in new drug development in prostate cancer to come together and release guideline statements from the Prostate-Specific Antigen Working Group. These guidelines focused on men with CRPC and defined eligibility, outcome measures and standardized the use of PSA in the context of clinical trials [26].
A number of trials studies evaluating docetaxel-based chemotherapy analyzed PSA response as a potential surrogate marker for survival [18, 27]. In another clinical setting a large US Intergroup study evaluated the role of continuous versus intermittent androgen deprivation therapy (ADT) in men with de-novo metastatic prostate cancer exploring the impact of the degree of PSA nadir on overall survival following 7 months of ADT [28, 29]. In that study while the median overall survival for men with nadir PSA values <0.2 ng/mL at 7 months of induction ADT was 75 months, patients with PSA nadir of 0.2–4.0 and >4 mg/mL had a median survivals of 44 and 13 months, respectively (p < 0.001). These results support the utility of nadir PSA as a potential surrogate endpoint in this clinical setting. Although nadir PSA is not an endpoint commonly employed in the castration-resistant setting, the association between PSA decline and outcome has been analyzed in contemporary trials. Secondary PSA analysis of TAX 327, a randomized phase III trial evaluating docetaxel plus prednisone given either every 3 weeks or weekly versus Mitoxantrone plus prednisone given every 3 weeks demonstrated a 60 % risk reduction of death in mCRPC patients achieving PSA declines greater than 50 % compared to their baseline PSA value [30]. In contrast, SWOG 9916, a similar randomized phase III study evaluating docetaxel-based chemotherapy in mCRPC examined the impact of the percentage of PSA decline on median overall survival. To determine whether PSA decline could be utilized as a surrogate marker for outcome, a Cox model testing for significance between treatment and survival time after adjustment for PSA decline was developed. PSA declines of 10, 20, 30, 40, 60, 75, 80, and 90 % were considered. After adjusting for a post-treatment PSA decline of 30 %, the treatment effect was no longer significant. While these results suggested that a PSA decline ≥30 % during treatment with docetaxel-based chemotherapy could be used as a surrogate marker for survival, PSA decline by itself could not fully explain total dependence between treatment received and the observed survival outcome [31].
While the utility of PSA response remains undefined, it is a rationale target in the context of AR directed therapeutics. Agents such radium-223 and Sipuleucel-T and a number of novel agents in development however do not appear to block AR transcription and thus the lack of impact on PSA decline. In studies of these agents, PSA is a far less attractive biomarker for activity. Taking this and other response questions into consideration the role of PSA, PSA-related measures, and other outcome measures for trials in mCRPC were reconsidered by the Prostate Cancer Clinical Trials Working Group (PCWG2) [32]. Recommendations from this group included an increased emphasis on time-to-event endpoints (i.e., failure to progress) when moving from phase II to III trials. Likewise, PCWG2 recommended that PSA should not be used to define treatment efficacy nor as a criteria for drug discontinuation in individual patients or studies. Contrary to the recommendations from PCWG1 regarding the reporting of PSA response rates, PCWG2 recommended against such actions as serologic responses appears to have little value given the uncertain significance of a defined degree of decline from baseline and the absence of prospective evidence of its utility as a surrogate of clinical benefit. PCWG2 did however recommend that in the design of mCRPC studies, the percentage of change in PSA from baseline to 12 weeks (including those who discontinue therapy earlier), as well as the maximum decline in PSA that occurs at any point after treatment be reported for each patient in the form of a waterfall plot [32]. These recommendations are now widely accepted and have been incorporated in the design of modern trials evaluating agents such as cabazitaxel, Sipuleucel-T, abiraterone acetate (AA), radium-223, and enzalutamide [7, 9, 11, 33].
Recently, Halabi and colleagues analyzed PSA data from the phase III study evaluating cabazitaxel versus mitoxantrone and prednisone in mCRPC patients previously treated with a docetaxel-containing regimen [34]. The primary aim of their analysis was to confirm previous findings from SWOG 9916 about the potential surrogacy of a PSA decline (≥30 %) within 12 weeks of treatment initiation with docetaxel-based chemotherapy. When Prentice operational criteria were applied, the treatment received was a statistically significant predictor for overall survival [35]. Similarly, a PSA decline of >30 % was also a significant predictor for overall survival with a hazard ratio of 0.52 (p < 0.001). Unfortunately, in the multivariable model both PSA decline and individual treatment arm remained statistically significant thus failing to meet criteria for surrogacy. When using Prentice criteria, a marker is considered a surrogate endpoint if it is statistically significantly associated (p < 0.05) with overall survival in both univariate models. However, in the multivariable model, the marker but not treatment arm needs to be statistically significant. Furthermore, the proportion of treatment effect explained for PSA decline of ≥30 and 50 % in this report did not exceed 0.50, the lower bound of the 95 % CI, suggesting a lack of surrogacy [34].
Significant PSA responses were also observed in the mCRPC trials with the CYP-17 inhibitor abiraterone acetate. While in the post-docetaxel study a PSA response was observed in 29.5 % of patients, almost 62 % of patients in the chemotherapy-naive study achieved a PSA decline of ≥50 % [8, 9]. Similarly, the PSA responses observed in the post-chemotherapy enzalutamide phase III trial and the enzalutamide chemotherapy-naive phase III study were 54 and 78 %, respectively [7, 36]. Despite the impressive serologic responses observed in these studies, the utility PSA decline as a surrogate endpoint remains undefined.
Limitations of PSA Endpoints
As we have described, PSA response has failed to meet requirements for surrogacy for survival in trials evaluating cytotoxic therapy, yet PSA testing remains a critical parameter for practicing physicians and their patients. While PSA values in the individual patient is almost certainly ordered too often and frequently used to make therapeutic decisions in routine clinical practice irrespective of the clinical setting, it is this routine and uncontrolled practice that has affected the integrity and outcome of many US-based prostate cancer studies. Although PSA declines in patients receiving chemotherapy are highly prognostic and perhaps an easy measure to evaluate response within a few months of initial treatment, there is no cut point that can be used to fully predict benefit for an individual patient. Similarly, transient elevations of PSA while receiving novel agents could be the result of a “flare phenomenon” and not representative of true disease progression [32]. Early recognition of this phenomenon is critical to avoid early drug discontinuation in routine practice and clinical trials.
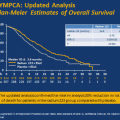
Whether PSA can be used as a biomarker for assessing anti-tumor activity and disease outcome when evaluating novel non-AR targeting agents remains unknown. Two recent, large, randomized phase III studies evaluating active agents in mCRPC highlight the disconnect that exists between PSA and overall survival. The IMPACT trial (Immunotherapy for Prostate Adenocarcinoma Treatment), a phase III, double-blind, placebo-controlled study enrolled 512 men with asymptomatic or minimally symptomatic mCRPC randomizing them 2:1 to receive sipuleucel-T or placebo [11]. Treatment with sipuleucel-T prolonged median overall survival, the primary endpoint, by 4.1 months compared to placebo (25.8 versus 21.7 months, respectively) and reduced the risk of death from any cause by 22.5 % (p = 0.032). The significant improvement in overall survival in men with symptomatic or minimally symptomatic metastatic CRPC in men receiving sipuleucel-T was obtained without evidence of a measurable anti-tumor effect such as objective response in soft tissue or PSA reduction of at least 50 %. These results have added to the controversy regarding the role and utility of this novel immunotherapy in mCRPC [37].
More recently the alpha-emitter, radium-223 demonstrated an overall survival improvement in men with mCRPC with predominant bone metastases [33]. In addition to the statistically and clinical relevant survival benefit radium-223 delayed the development of symptomatic skeletal events (SSEs). Of interest however is that these clinically meaningful endpoints were obtained with minimal impact on PSA. Although not surprising based on its presumed mechanism of action, the lack of association between PSA decline and survival provides additional evidence of the need to move away from PSA endpoints in this patient population. Although PSA progression defined by either PCWG1 or 2 has been removed from contemporary CRPC trial designs, treatment discontinuation secondary to rising PSA while on therapy remains a major issue in routine clinical practice. Although retrospective analyses of both docetaxel trials (TAX327 and SWG9916) suggested that an increase in PSA at 3 months of therapy correlates with poor overall survival, to date the utility of PSA progression in the castration-resistant setting has not been prospectively validated and remains undefined.
Objective Response
Tumor burden reduction has been the most traditional method used to assess efficacy in cancer therapeutics. Phase II studies in many solid tumors assess the effect and duration of a treatment using response rates defined by Response Evaluation Criteria in Solid Tumors (RECIST) [38]. Although no prostate cancer therapeutic has been FDA approved solely on this basis, objective response assessment is typically included in phase II mCRPC trial designs. With the exception of sipuleucel-T and radium-223, all of the agents FDA approved for mCRPC demonstrated a degree of objective response in phase III testing. Given the bone tropism of advanced PCa and the lack of objective means to assess response in bone the utility of objective response as a means of assessing therapeutic benefit in mCRPC has been somewhat limited. In most trials conducted in the current era, only 35–40 % of patients with mCRPC have overt evidence of lymph node metastases while less than 5–8 % of patients present with visceral disease [39].
Following the results of the two major phase III trials of docetaxel, efforts to interrogate the potential for ORRs to provide some degree of surrogacy for survival have been explored. In the TAX 327 trial, patients randomized to every 3 weeks docetaxel arm demonstrated an ORR of 12 % while those receiving docetaxel plus estramustine in SWOG 9916 manifested a 17 % response rate [30, 31]. A subsequent report by the TAX327 investigators analyzed the association of measurable tumor response with survival. Tumor response was evaluated by World Health Organization (WHO) criteria, for which the product of the largest diameter and its perpendicular is summed for predefined lesions.
Of the 1,006 patients enrolled on the TAX 327 study, 412 (41 %) had measurable disease. Of those, 37 patients exhibited an objective response (CR/PR, 9.0 %). Partial responders demonstrated longer median overall survival (29.0 months) than patients with stable or progressive disease, with these findings remaining after evaluation with a landmark analysis [40]. While all patients with measurable tumors who achieved an objective response exhibited PSA declines, only a proportion of patients with PSA declines exhibited objective responses. Although this data suggests that an objective response by WHO criteria in men receiving docetaxel for mCRPC could translate into a survival benefit, the clinical relevance of this finding in the setting of widely metastatic bone disease remains unclear.
Recently developed AR targeted agents have demonstrated objective responses in those patients with measurable disease. When evaluating the response rate of abiraterone plus prednisone in men with measurable disease participating in the post-docetaxel abiraterone phase III trial, 14 % of patients in the abiraterone arm compared to 3 % of patients in the prednisone plus placebo arm achieved a RECIST-defined PR or CR (p < 0.001) [8]. In the chemotherapy-naïve trial, patients receiving abiraterone plus prednisone demonstrated an ORR of 36 % compared to 16 % of patients receiving prednisone/placebo (p < 0.001) [9]. It is important to recognize that in contrast to the older docetaxel-based chemotherapy studies, the abiraterone phase III trials utilized RECIST criteria which incorporates a different criteria for nodal disease response assessment [38]. Perhaps the greatest number of patients with measurable disease ever enrolled in an mCRPC trial was the post-docetaxel trial of enzalutamide, a randomized, double-blind, placebo-controlled study which randomized patients with mCRPC who had previously been treated with one or two chemotherapy regimens to receive enzalutamide or placebo. In this trial over 92 % of patients receiving enzalutamide had bone metastases, among them almost 40 % had extensive disease (>20 bone lesions). Soft tissue disease was evaluable in over 70 % of patients. Lymph node metastasis was present in approximately 55 % of patients while visceral liver and lung disease was seen in 11 and 15 % of patients, respectively. The ORR reported was 29 versus 4 % in favor or enzalutamide therapy (p < 0.001) [7]. Recent results from the chemotherapy naïve phase III trial of enzalutamide (PREVAIL) reported a response rate of 58.8 % in patients treated with enzalutamide in contrast to a. 4.9 % in the placebo control arm (p < 0.0001). Of interest is that 20 % of patients achieved a RECIST-defined CR [36]. Both studies evaluating enzalutamide (pre and post-chemotherapy) used RECIST 1.1 to measure tumor response. Some of the important changes made in RECIST 1.1 criteria include: (a) reduction in the number of lesions to be assessed (from a maximum of ten to a maximum of five total; (b) number of lesions per organ also down to two, maximum); (c) lymph nodes are now considered measured lesions if larger than 1.5 cm in short axis; (d) confirmation of response is required for trials with ORRs as the primary endpoint but not in randomized studies since the control arm serves as appropriate means of interpretation of data; (e) in addition to the 20 % standard increase for progressive disease, a 5 mm absolute increase is also mandated [41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree