Decreased Vitality: Introduction
Endocrine Disease
Approximately 50% of older people have glucose intolerance with normal fasting blood sugar levels. Although poor diet, obesity, and lack of exercise may account for some of these findings, aging itself is associated with deteriorating glucose tolerance, primarily attributable to a change in peripheral glucose utilization, although beta-cell dysfunction and decreased insulin secretion are also contributing factors. Glucose intolerance alone is not sufficient to diagnose diabetes mellitus. However, such individuals are at increased risk of developing diabetes mellitus. Prediabetes is identified as impaired fasting glucose (fasting plasma glucose level of 100-125 mg/dL), as impaired glucose tolerance (plasma glucose level of 140-199 mg/dL 2 hours after 75 g of glucose), or as a glycosylated hemoglobin of 5.7% to 6.4% (diagnosis of diabetes and prediabetes is reviewed in Inzucchi, 2012). Lifestyle modification, including weight loss and exercise, prevents or forestalls the development of type 2 diabetes in individuals with glucose intolerance (Diabetes Prevention Program Research Group, 2002; reviewed in Gillies et al., 2007). Both the U.S. Preventive Services Task Force (USPSTF) and the American Diabetes Association (ADA) recommend these interventions for patients at risk for diabetes (U.S. Preventive Services Task Force, 2003).
More than 25% of individuals age 65 and older in the United States have diabetes mellitus (Ligthelm et al., 2012). Many of these patients are unaware that they have the disease. The USPSTF concludes that the evidence is insufficient to recommend for or against routinely screening asymptomatic adults for type 2 diabetes, except that those with hypertension or hyperlipidemia should be screened as an approach to reducing cardiovascular risk. The ADA recommends that screening should begin at age 45 at 3-year intervals, but at shorter intervals in high-risk patients. The diagnosis of diabetes should be made based on a fasting plasma glucose level of ≥126 mg/dL or a glycosylated hemoglobin of ≥6.5% and confirmed by either test. Initial evaluation in patients with type 2 diabetes should include glycosylated hemoglobin level, fasting lipid profile, basic metabolic panel, urine dipstick for overt proteinuria or screen for microalbuminuria, and electrocardiography.
The therapeutic goal for relatively healthy, nonfrail older diabetic patients is the same as that for younger patients: normal fasting plasma glucose without hypoglycemia. According to the American Geriatrics Society, the Canadian Diabetes Association, the ADA, and the European Diabetes Working Party, a hemoglobin A1c (HbA1c) value of 7% or less is reasonable for healthy and well-functioning adults. However, in frail individuals and those with short life expectancies (including many nursing home residents), the therapeutic goal may be modified to eliminate symptoms associated with hyperglycemia, to reduce hypoglycemia, and to enhance quality of life. This can be accomplished by lowering the blood sugar to levels that avoid glycosuria. A less restrictive HbA1c (<8%) may be more appropriate for these patients. The age at which tight control is no longer indicated has not been defined; however, studies that demonstrated benefit of tight control were 4 to 6 years in duration. Among older diabetic patients, the presence of multiple comorbid illnesses or functional impairments is a more important predictor of limited life expectancy and diminishing expected benefits of intensive glucose control than is age alone (Huang et al., 2008). Although glycemic control may provide benefit in many older individuals, the greater risk of hypoglycemia in older adults must be considered when managing diabetes mellitus in this population (Ligthelm et al., 2012). Large randomized trials in patients with type 2 diabetes suggest that tight glycemic control burdens patients with complex treatment programs, hypoglycemia, weight gain, and costs and offers uncertain benefit in return (Montori and Fernandez-Balsells, 2009). There is also an association between tight glycemic control (HbA1c <7%) and a greater risk of hip fracture (Puar et al., 2012). In community-dwelling, nursing home–eligible individuals with diabetes mellitus, HbA1c of 8.0% to 8.9% is associated with better functional outcomes at 2 years than HbA1c of 7.0% to 7.9%, suggesting that even the current American Geriatrics Society guideline recommending an HbA1c target of 8% or less for older adults with limited life expectancy may be lower than necessary to maintain function (Yau et al., 2012).
Because most patients with adult-onset diabetes are obese, weight reduction should be attempted, although only approximately 10% will maintain a prolonged weight loss. Dietary fats should be reduced. Aerobic exercise can both delay the onset of type 2 diabetes mellitus and improve insulin resistance in individuals with established disease (Boulé et al., 2001). A combination of aerobic and resistance training is effective in lowering HbA1c levels in patients with type 2 diabetes mellitus (Church et al., 2010).
Several groups of oral hypoglycemic agents, each with a different mechanism of action, are now available. Comparative effectiveness and safety of medications for type 2 diabetes and clinical practice guidelines have been published (Bennett et al., 2011; Qaseem et al., 2012). Sulfonylureas act primarily by increasing beta-cell insulin secretion. They increase circulating insulin levels and are frequently associated with weight gain and may cause hypoglycemia. HbA1c reduction is in the 1% to 2% range. Although package inserts carry a warning on the increased risk of cardiovascular disease, the United Kingdom Prospective Diabetes Study (UKPDS) Group reported no adverse cardiovascular outcomes in sulfonylurea-treated patients (United Kingdom Prospective Diabetes Study Group, 1998a).
Acarbose reduces postprandial plasma glucose by inhibiting small intestine brush-border α-glucosidases. It has a small effect on metabolic control (HbA1c reduction of 0.5%-1%) and causes frequent gastrointestinal distress with bloating and flatulence.
Metformin, a biguanide, exerts its major metabolic effect by inhibiting hepatic glucose production. Metformin leads to significant improvement in glucose control when used alone (HbA1c reduction of 1%-2%) or in combination with a sulfonylurea (Inzucchi et al., 1998). Unlike sulfonylureas, which often lead to weight gain, metformin therapy is associated with weight loss, a benefit to most type 2 diabetic patients. Weight should be monitored closely in thin diabetic patients. A serious side effect of metformin is lactic acidosis. It should not be used in patients with renal insufficiency or chronic heart failure. In patients age 80 years or older, creatinine clearance should be measured before therapy is initiated. Metformin should be discontinued during illnesses associated with volume depletion and prior to surgery.
Thiazolidinediones lower blood sugar by improving target-cell insulin sensitivity. Besides the potential for hepatic toxicity, which requires frequent liver function testing, thiazolidinedione therapy may be associated with marked weight gain. Fluid retention occurs more frequently in combination with insulin and in heart failure, making these conditions contraindications for the use of these agents. The thiazolidinedione, rosiglitazone, is associated with an increased risk of heart failure, acute myocardial infarction, and death (Lipscombe et al., 2007; Singh, Loke, and Furberg, 2007). This appears to be limited to rosiglitazone because, although pioglitazone is associated with an increased risk for serious heart failure, it is not associated with increased risk for death, myocardial infarction, or stroke (Lincoff et al., 2007).
Meglitinide analogues (nateglinide and repaglinide) are nonsulfonylurea insulin secretagogues with shorter onset of action and half-life than sulfonylureas, a greater decrease in postprandial glucose level, and a lower risk of hypoglycemia. They may be good choices for individuals with irregular mealtimes but are more expensive than other oral diabetes drugs, and morbidity and mortality effects are unknown (reviewed in Black et al., 2007).
Two other classes of agents have been approved: incretin mimetics and dipeptidyl peptidase IV (DPP-4) inhibitors (reviewed in Amori, Lau, and Pittas, 2007). The first incretin mimetic, exenatide, has the same glucoregulatory action as glucagon-like peptide-1 (GLP-1), a naturally occurring incretin hormone (reviewed in Drucker, 2007). It enhances glucose-dependent insulin secretion, suppresses inappropriately high glucagon secretion, slows gastric emptying, decreases food intake, promotes beta-cell proliferation and neogenesis, reduces adiposity, and increases insulin-sensitizing effects. It is administered by subcutaneous injection twice daily, with a major side effect of nausea and vomiting. Adding twice-daily exenatide injections improved glycemic control without increased hypoglycemia or weight gain in patients with uncontrolled type 2 diabetes who were receiving insulin glargine treatment (Buse et al., 2011). In 2006, the U.S. Food and Drug Administration (FDA) approved sitagliptin, a DPP-4 inhibitor. By inhibiting the breakdown of GLP-1 and glucose-dependent insulinotropic polypeptide, it leads to a lower blood glucose, stimulates insulin secretion, and decreases levels of circulating glucagon. It does not inhibit gastric emptying or promote satiety and does not lead to weight loss. It is well tolerated without nausea or vomiting. Sitagliptin is one of the less effective glycemia-lowering drugs (HbA1c reduction of 0.5%-0.9%) (Nathan, 2007).
Table 12–1 presents a step-care approach to the treatment of type 2 diabetes. Because of the results of the UKPDS study, close control of blood glucose in type 2 diabetes is now in vogue. Intensive blood glucose control with sulfonylureas or insulin decreased the risk of microvascular complications but not macrovascular disease. Intensive treatment did increase the risk of hypoglycemia. Intensive blood glucose control with metformin in overweight patients decreased the risk of both microvascular and macrovascular complications and was associated with less weight gain and fewer hypoglycemic attacks than insulin and sulfonylureas (United Kingdom Prospective Diabetes Study Group, 1998b). Metformin is recommended as first-line pharmacological therapy in overweight patients. It is important to observe patients closely for hypoglycemic reactions and their ability to respond to this stress if any of these regimens is prescribed. Although each agent is effective as monotherapy, the majority of patients need multiple therapies to attain glycemic target levels in the longer term.
Step | Criteria and recommendations | Monitoring |
|---|---|---|
Step 1: Evaluation and nonpharmacologic approaches | Newly diagnosed or current therapy ineffective: initiate or reinforce diet, exercise, home blood glucose monitoring, formal diabetes education Current therapy effective: continue therapy | Success: continue step 1 Failure after 2-3 months: go to step 2* |
Step 2: Oral monotherapy | Obese or dyslipidemic: first line— metformin; second line—thiazolidinedione or acarbose Nonobese: first line—sulfonylurea or metformin; second line—acarbose | Success: continue step 2 Failure: go to step 3 |
Step 3: Oral combination therapy | Previously on metformin: add sulfonylurea Previously on sulfonylurea: add metformin or thiazolidinedione Previously on thiazolidinedione: add sulfonylurea Previously on acarbose, obese: add metformin Previously on acarbose, not obese: add sulfonylurea | Success: continue step 3 Failure: go to step 4 |
Step 4: Insulin initiation or insulin + oral therapy | FBG <200-240: eliminate one drug and always thiazolidinedione, start bedtime glargine FBG >200-240: eliminate one drug, start bedtime glargine and preprandial lispro† | Success: continue step 4 Failure: consult specialist |
Patients who do not achieve adequate glycemic control with a combination of oral medications are candidates for insulin treatment. Insulin therapy may be combined with oral therapy to better achieve target glucose control. Glucose monitoring is essential for titrating the insulin dose. Multiple insulin regimens are possible, but most begin with a basal insulin of once-daily glargine or twice-daily neutral protamine Hagedorn (NPH) insulin. Insulin glargine has modestly better glycemic benefits than NPH insulin with a low risk of hypoglycemia (Lee et al., 2012). Fast-acting insulin may be added with meals to attain better glycemic control (reviewed in Mooradian, Bernbaum, and Albert, 2006; Holman et al., 2009).
Pramlintide has been approved for use with mealtime insulin. It is a synthetic form of the hormone amylin, which is produced and secreted along with insulin by pancreatic beta-cells. It enhances insulin action and is administered subcutaneously before each meal. Again, as with other new agents, there are no long-term morbidity or mortality data.
A new agent, dapagliflozin, a selective inhibitor of sodium-glucose cotransporter 2, improves glycemic control, stabilizes insulin dosing, and reduces weight without increasing major hypoglycemic episodes in patients with inadequately controlled type 2 diabetes mellitus (Wilding et al., 2012). Currently, there are no specific data on this agent in older adults.
Diabetics are at increased risk of macrovascular disease. Other atherosclerotic risk factors such as smoking, dyslipidemia, and hypertension should be eliminated or treated. Therapy of these risk factors may be of greater benefit than tight glucose control in older diabetics. Blood pressure control reduces both micro- and macrovascular complications in type 2 diabetes (United Kingdom Prospective Diabetes Study Group, 1998c). There is no evidence from randomized trials to support a strategy of lowering systolic blood pressure below 135 to 140 mm Hg in persons with type 2 diabetes mellitus (Cushman et al., 2010). A meta-analysis of cholesterol-lowering and blood pressure–lowering trials demonstrated large, significant effects on reducing macrovascular disease in type 2 diabetes (Huang, Meigs, and Singer, 2001). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) attenuate progression of nephropathy in both type 1 and type 2 diabetic patients with hypertension and in normotensives with microalbuminuria. They also attenuate decline in renal function in normotensive, normoalbuminuric type 2 diabetic patients (reviewed in Strippoli et al., 2006). The antihypertensive regimen of diabetics should include an ACE inhibitor or ARB, and such therapy should be initiated in normotensive albuminuric patients. Intensified multifactorial intervention with tight glucose regulation and the use of renin–angiotensin system blockers, aspirin, and lipid-lowering agents has been shown to reduce the risk of nonfatal cardiovascular disease and the rates of death from cardiovascular causes and death from any cause (Gaede et al., 2008).
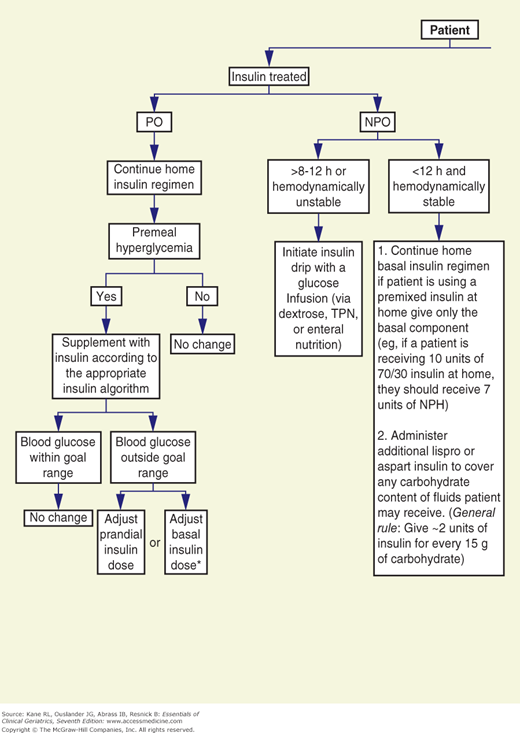
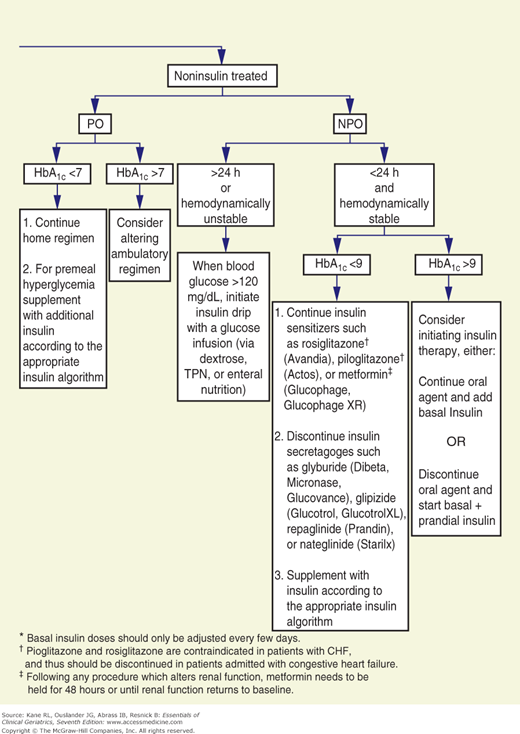
The goals for glycemic control are less well established for hospitalized than for ambulatory patients. However, data suggest that maintaining blood glucose at 80 to 110 mg/dL in critically ill patients reduces mortality and that hyperglycemia adversely affects wound healing and increases the risk for infection (reviewed in Inzucchi, 2006). Although sliding scale insulin regimens are frequently used in hospitalized patients with diabetes, it is the opinion of the authors that they should not be used. The University of Washington has developed an algorithm to replace sliding scale insulin orders (Fig. 12-1).
Figure 12-1
Flow diagram for treatment of hospitalized (nonintensive care unit) patients with type 2 diabetes mellitus. CHF, congestive heart failure; NPH, neutral protamine Hagedorn (insulin); NPO, nothing by mouth; PO, by mouth; TPN, total parenteral nutrition. (Reproduced with permission from Ku, 2002.)
Older adults have an increased incidence of hyperosmolar nonketotic (HNK) coma. Characteristic symptoms and signs help the physician distinguish this syndrome from diabetic ketoacidotic (DKA) coma. Table 12–2 compares HNK and DKA. Whereas DKA frequently develops over hours, HNK typically develops over days to weeks. Focal or generalized seizures are common in HNK and unusual in uncomplicated DKA. The fluid deficit is greater in HNK, thus leading to a higher serum sodium and more marked rise in blood urea nitrogen. Therapy in HNK must therefore address the volume and hyperosmolar state of the patient. Because these patients may be quite sensitive to insulin, lowering of glucose should be done cautiously. Volume replacement should be initiated with normal saline. This therapy alone may reduce blood glucose levels, as renal perfusion is enhanced and glucose is lost in the urine. If, after 1 hour of volume repletion, blood glucose levels are not reduced, a bolus of 20 U of regular insulin should be administered intravenously. If glucose levels do not respond, an insulin drip may be started. Such an approach should allow repletion of volume without lowering serum osmolarity too rapidly.
HNK* | DKA | |
|---|---|---|
Time of development | Days to weeks | Hours |
Seizures | Common | Uncommon |
Fluid deficit | Marked | Present |
Serum sodium | ↑↑ | ↑ |
Blood urea nitrogen | ↑↑ | ↑ |
The changes with aging in the hypothalamic-pituitary-thyroid axis have been reviewed (Habra and Sarlis, 2005). Although thyroid function is generally normal in aging, clinicians should be aware of the norms for thyroid function tests for this age group (Table 12–3). The majority of data indicate that thyroxine (T4) levels are normal. Triiodothyronine (T3) levels may be lower in healthy older people when compared to younger individuals but are still in the normal range. It has been suggested that the lower T3 levels reported in several studies are caused by undiagnosed illness and the low T3 syndrome described later. Thyroid-stimulating hormone (TSH) levels are also normal, whereas the TSH response to thyroid-releasing hormone (TRH) is decreased in males and normal in females. Thus, the TRH test is less valuable in older males. Metabolic clearance of thyroid hormones is decreased in aging. With intact feedback loops, normal thyroid function is maintained despite this change. However, with exogenous replacement of thyroid hormone, such regulatory mechanisms are not maintained; thyroid replacement doses in older adults should be lower to take into account the lower metabolic clearance. Laboratory evaluation tests most useful in thyroid disease are summarized in Table 12–4.
Hypothyroidism | Hyperthyroidism | |
|---|---|---|
T4 | E | E |
TSH | E | E |
Free T4 | E | E |
T3 | O | D |
Radioactive iodine uptake | O | D |
TRH test | D (Females) | D (Females) |
O (Males) | O (Males) | |
Reverse T3 | D | D |
TSH stimulation | D | O |
T3 suppression | O | O |
Hypothyroidism is primarily a disease of those age 50 to 70 years. Goiter is rarely seen with hypothyroidism in older adults except when it is iodide induced. Diagnosis is usually made by a low free T4 and an elevated TSH. Because total T4 levels may be depressed in seriously ill patients, diagnosis of hypothyroidism should not be made on the basis of low T4 levels alone. Table 12–5 lists laboratory characteristics of the low T4 syndrome associated with nonthyroidal illness. Not all free T4 methods distinguish the low T4 syndrome from hypothyroidism; physicians should be aware of the type of determination and interpretation used in their laboratory. Because the T3 level may be in the normal range in hypothyroidism, this is not a helpful test. The low T3 level associated with a host of acute and chronic nonthyroidal illnesses also contributes to the poor specificity of this test in hypothyroidism. Approximately 75% of circulating T3 is derived from peripheral conversion from T4. The enzymes that convert T4 to T3 or reverse T3 are under metabolic control. During illness, more T4 is converted to reverse T3, leading to the characteristic laboratory findings of the low T3 syndrome.
Low T4 syndrome | Low T3 syndrome | |
|---|---|---|
T4 | Decreased | Normal |
Free T4 | Normal or increased | Normal |
T3 | Decreased | Decreased |
Reverse T3 | Normal or increased | Normal or increased |
Thyroid-stimulating hormone | Normal | Normal |
The radioactive iodine uptake is also not helpful because normal values are so low that they overlap with hypothyroidism. The TRH stimulation test can be used in females, but decreased responsiveness to TRH in older males does not allow this test to distinguish normal from pathologic states. In males, a TSH stimulation test may help to confirm the presence of hypothyroidism.
Hypothyroidism may be accompanied by other laboratory abnormalities. Creatine phosphokinase (CPK) levels may be elevated. A normocytic, normochromic anemia, which responds to thyroid hormone replacement, may be present. There is an increased incidence of pernicious anemia in hypothyroidism, but the microcytic anemia of iron deficiency remains the most common anemia associated with hypothyroidism.
The symptoms and signs of hypothyroidism may be overlooked when such complaints as fatigue, memory loss, and decreased hearing are ascribed to aging without further investigation. The prevalence of undiagnosed hypothyroidism in healthy older people has varied from 0.5% to 2% in multiple studies; consequently, a general screening program is not cost-effective. The prevalence among older adults who are ill, however, is sufficient to support screening for hypothyroidism in this population, comprising individuals who have already presented themselves for care.
Therapy for hypothyroidism should be started at 0.025 to 0.05 mg of sodium levothyroxine (Synthroid) per day and increased by the same dose at 1- to 3-week intervals. The decreased metabolic clearance rate of thyroid hormone in aging may lead to a lower maintenance dose of T4. The physician should monitor heart rate response and symptoms of angina and, in the laboratory, the TSH level. When indicated for symptomatic cardiovascular disease, a β-blocker may be added to the T4 regimen. In patients with coronary artery disease, therapy can be initiated with T3 5 μg/day and increased by 5 μg at weekly intervals to a level of 25 μg/day, at which time the patient can be converted to T4 therapy. Because T3 has a shorter half-life than T4, symptoms will remit more rapidly after discontinuance of therapy if the patient develops cardiovascular complications. A β-blocker can also be added to the T3 regimen. T4 monotherapy, rather than T4-T3 combination, should remain the treatment of choice for clinical hypothyroidism (Grozinsky-Glasberg et al., 2006).
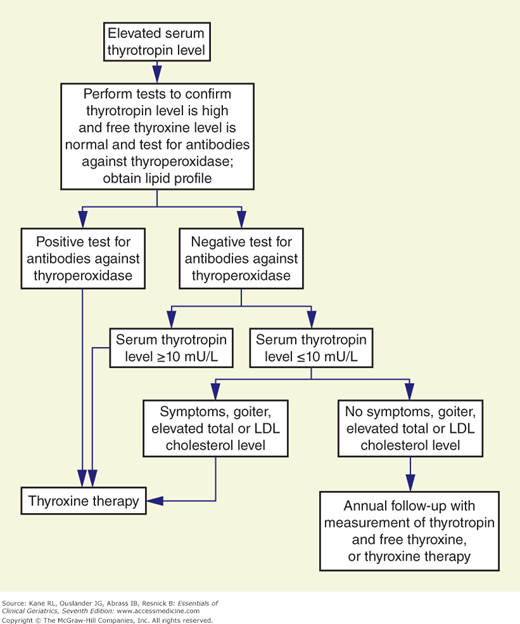
Subclinical hypothyroidism is characterized by increased serum TSH concentrations with normal free T4 and free T3 levels. It occurs in 10% to 15% of the general population. The presentation is nonspecific, and symptoms are usually subtle. In a prospective follow-up study, based on the initial TSH level (4 to 6, >6 to 12, or >12 mIU/L), the incidence of overt hypothyroidism after 10 years was 0%, 42.8%, and 76.9%, respectively (Huber et al., 2002). The incidence of overt disease was increased in those with positive microsomal antibodies. Subclinical hypothyroidism is associated with an increased risk of coronary heart disease (CHD) events and CHD mortality in those with higher TSH levels, particularly in those with a TSH concentration of 10 mIU/L or greater (Rodondi et al., 2010), and is associated with left ventricular diastolic dysfunction that is improved with T4 therapy (Biondi et al., 1999). Others have not found an association of subclinical hypothyroidism with cardiovascular disorders or mortality (Cappola et al., 2006). The decision to treat patients remains controversial (Fatourechi, 2007). Contributing to this controversy is the question of the age-specific distribution of TSH (Surks and Hollowell, 2007). This is further complicated by data showing reduced mortality risk in untreated mild hypothyroid subjects age >85 years (Mariotti and Cambuli, 2007). Despite the controversy, some experts favor treating most patients because (1) some studies do report improvement in some outcomes (eg, cognition, lipids, and heart function); (2) the risks of thyroid replacement therapy are low; (3) the cost of thyroid replacement therapy is low; and (4) it will prevent the development of symptoms of clinical hypothyroidism, which occurs in 5% of patients per year (Gharib, 2008). If therapy is contemplated, an algorithm for the management of subclinical hypothyroidism has been proposed (Fig. 12-2).
Figure 12-2
An algorithm for the management of subclinical hypothyroidism. LDL, low-density lipoprotein. (Reproduced with permission from Cooper, 2001.)
Most patients with myxedema coma are older than age 60 (Table 12–6). In approximately 50% of the cases, the coma is induced in the hospital by treating hypothyroid patients with hypnotics. A neck scar, from previous thyroid surgery, is a clue to the cause of coma. Because patients with this disorder die of respiratory failure, hypercapnia requires prompt attention. These patients should be treated in an intensive care setting, with intubation and respiratory assistance instituted at the first sign of respiratory failure. The cerebrospinal fluid protein level is often more than 100 mg/dL and should not in itself be used as an indicator of other central nervous system pathology. Therapy includes an initial dose of T4 intravenously. Although studies have not been done to demonstrate the efficacy of glucocorticoids in this syndrome, it is generally recommended that these patients receive 50 mg of hydrocortisone every 6 hours for the first 1 or 2 days. Patients with concomitant adrenal insufficiency will require continued steroid therapy.
Approximately 20% of hyperthyroid patients are older adults; 75% have classic signs and symptoms. Ophthalmopathy is infrequent. Approximately one-third have no goiter. Toxic multinodular goiter is more frequent in older persons than in the young. Severe nonthyroidal disease may disguise thyrotoxicosis (apathetic hyperthyroidism). Congestive heart failure (CHF), stroke, and infection are common disorders associated with masked hyperthyroidism. There should be a high threshold of suspicion for hyperthyroidism in older adults. Unexplained heart failure or tachyarrhythmia, recent onset of a psychiatric disorder, or profound myopathy should raise questions about masked hyperthyroidism. The triad of weight loss, anorexia, and constipation, which may raise the possibility of neoplastic disease, occurs in 15% of older thyrotoxic patients. Diagnosis is made by T4, T3, and/or radioactive iodine uptake (see Table 12–4). The ultrasensitive TSH assays can differentiate hyperthyroidism from normal. In the absence of acute nonthyroidal disease, this test alone may confirm the clinical diagnosis of hyperthyroidism. In the presence of acute illness, concomitant determination of TSH and free T4 may be more appropriate. A T3 suppression test should not be done in older adults because of the risk of angina or myocardial infarction.
Therapy is usually by radioactive iodine ablation. Often patients are first treated with antithyroid medications to control hyperthyroidism and deplete the thyroid gland of hormone prior to the radioactive iodine treatment (reviewed in Cooper, 2005). Surgery is reserved for patients with thyroid glands that are causing local obstructive symptoms.
Severe thyrotoxicosis is treated with antithyroid drugs (preferably propylthiouracil because it blocks peripheral conversion of T4 to T3) to inhibit new hormone synthesis, iodides to block thyroid hormone secretion, and β-blockers to decrease the peripheral manifestations of thyroid hormone action. In older adults with underlying cardiac disease, β-blocker therapy may be a problem; thus the cardiovascular response must be closely monitored. In patients allergic to antithyroid medications or where β-blockers are contraindicated, calcium ipodate (Oragrafin), 3 g every 3 days, can be used because it inhibits peripheral conversion of T4 to T3. Dexamethasone 2 mg every 6 hours inhibits peripheral conversion of T4 to T3 and may be added to any of the above regimens.
For the management guidelines of hyperthyroidism and other causes of thyrotoxicosis from the American Thyroid Association and the American Association of Clinical Endocrinologists, see Bahn et al. (2011).
Subclinical hyperthyroidism is defined as a combination of serum TSH of less than 0.45 mIU/L and normal serum free T4 and T3. Subclinical hyperthyroidism is associated with a 24% increase in mortality, a 29% increase in CHD mortality, and 69% incident atrial fibrillation in 9 years (Collet et al., 2012), and older subclinical hyperthyroid individuals are significantly more likely to have cognitive dysfunction than those who are euthyroid (hazard ratio, 2.26) (Ceresini et al., 2009). If the TSH level is less than 0.1 mIU/L, treatment is generally recommended with iodine-131 ablation. In patients with a TSH level of 1.0 to 4.5 mIU/L and atrial fibrillation, cardiovascular disease, or low bone density, or those with multinodular goiter, treatment is also recommended. If treatment is deferred, TSH and free T4 and T3 should be measured in 6 months.
One-third of patients with hyperparathyroidism are older than age 60. Symptoms are the same in older adults as in those who are younger but may be overlooked. Bone demineralization, weakness, and joint complaints may be ascribed to aging when they may actually indicate parathyroid disease. Primary hyperthyroidism is common in postmenopausal women with forearm fracture and low bone mineral density (Bergström, Landgren, and Freyschuss, 2007). Basic laboratory screening is indicated in these individuals. A National Institutes of Health Workshop has developed guidelines for surgery in patients with asymptomatic primary hyperthyroidism (Bilezikian et al., 2002, and reviewed in Marcocci and Cetani, 2011). Surgery is recommended if one of the following is present: serum calcium concentration greater than 1.0 mg/dL above upper limit of normal; 24-hour urinary calcium greater than 400 mg; creatinine clearance reduced by 30%; bone mineral density T-score lower than −2.5 at any site; and age less than 50 years. In those older than 50, surgery can be delayed with monitoring for the above criteria.
Table 12–7 contrasts some of the basic patterns of the common laboratory tests in hyperparathyroidism with those of other metabolic bone diseases that are common in older adults.
Disease | Ca | P | Alk | PTH |
|---|---|---|---|---|
Hyperparathyroidism | High | Low/normal | High/normal | High |
Osteomalacia | Low/normal | Low | High/normal | High |
Hyperthyroidism | High | High | High/normal | Low |
Osteoporosis | Normal | Normal | Normal | Normal/high |
Paget disease | Normal/high | Normal/high | High | Normal |