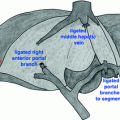
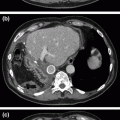
Fig. 2.1
Intraoperative picture of left lobe of liver in patient with metastatic small bowel NET to the liver. Large 8 cm lesion in inferior right lobe with other lesions in right lobe and caudate. Multiple small lesions in left liver make it clear that there is no curative option, and also demonstrates why right hepatectomy is not indicated due to extensive contralateral disease
An octreoscan was obtained and it showed activity in the hepatic metastasis; no activity in the small bowel mesentery, small bowel, or pancreas. Serum serotonin was elevated at 1,433 ng/mL (normal range 85–220). We performed upper endoscopy and colonoscopy, which showed no lesions. He had a capsule endoscopy which was negative.
He was seen in surgical consultation and was recommended to have an exploration with liver debulking, including intraoperative identification of his mid-gut primary and planned resection. He had been receiving monthly Sandostatin injections, and an intravenous Sandostatin drip was prepared for infusion as needed for carcinoid storm. The approach was right subcostal incision with extension to the left side. The lateral aspects of the subcostal incision was not curved superiorly as is typical for right hepatic lobectomy, but rather went more inferiorly to allow exploration of the abdominal cavity for his primary to facilitate exploration of the abdominal cavity for a primary lesion. The initial part of the operation was to assess the primary. It was found immediately on palpation of the distal small bowel which was in the pelvis. The hepatic flexure of the colon and area of the ileo-cecal valve was completely mobilized with some tethering of the primary lesion and palpable lymph nodes in a loop of distal small bowel mesentery in the pelvis. This distal small bowel was brought up into the subcostal incision and there was careful palpation of the bowel from the ligament of Treitz to the ileocecal valve. The solitary lesion approximately 8 cm proximal to ileocecal valve was the only mass palpated. This had not been visualized by colonoscopy.
For mid-gut NET that are more than 20% proximal to the ileocecal valve, every effort is made to try to preserve the ileocecal valve and do a segmental small bowel resection. At this site and with the location of the lymph node metastasis necessitating resection of the right colic trunk, a right hemicolectomy with resection of segment of small bowel and nodal metastasis was performed with standard anastomosis. Once the bowel resection and anastomsis was completed, the retractors were completely shifted from exposing the lower aspect of the abdomen to exposing the liver. The liver was completely mobilized and assessed by palpation and intraoperative ultrasound. The dominant segment 6 lesion was easily felt, there were four to five additional lesions in the right lobe > 1 cm and a large palpable caudate mass. The left and right liver had multiple small palpable metastases (Fig. 2.2). A cholecystectomy was performed. In this case, it was not necessary to remove the gallbladder to address any of the hepatic nodules, but for patients with NET with a laparoscopic approach, or certainly with an open approach liver metastasis, it is mandatory to remove the gallbladder as long-term use of Sandostatin will lead to formation of gallstones and ability to approach the gallbladder laparoscopically is compromised after such an extensive hepatic debulking procedure. To address the dominant lesion in the right segment 6, the right hepatic lobe was completely mobilized off the inferior vena cava. The feeding vasculature from the inferior segmental portal triad was assessed by surgeon-directed ultrasound and entered the lesion at the inferior medial border of this mass. The approach was to identify the margin of this large hepatic metastasis in a lateral avascular area. As is typical, it was firm, white, and once we were on the capsule either with blunt dissection with a finger or with a right angle, the surrounding parenchyma was swept away. When bridging vessels were seen, they were controlled with clips or Aquamontys ablation. Intraoperative ultrasound-guided dissection to where the main trunk was plastered over this and a vascular stapler was used to divide the main trunk. This large lesion was removed with very little surrounding parenchyma and very little blood loss (Fig. 2.3a). The cut parenchyma of the base was controlled with argon beam laser. All other small nodules were then addressed. Any nodule larger than 5 mm on the surface was resected with cautery and some exophytic lesions sharply resected with the base treated with cautery. Lesions just under the surface in the range of 1–3 cm had a circular incision made with cautery right over the palpable nodule. Once the white capsule of the nodule was identified, again blunt dissection either with the finger or right angle clamp was used to go around this often resecting 3 cm lesions in under 30 s. Several small 2–4 mm lesions were controlled with Aquamontys ablation on the surface with the typical popping noise (Fig. 2.3b). Ultrasound revealed two lesions, one in the caudate that was medially posterior to the main left segmental portal triad (Fig. 2.1) and a second lesion that was deep anterior to the right portal triad. An attempt was made to enucleate the caudate lesion, but it was too close to the main left portal structures. These were treated with radiofrequency ablation with ultrasound guidance following standard ablation algorithms.
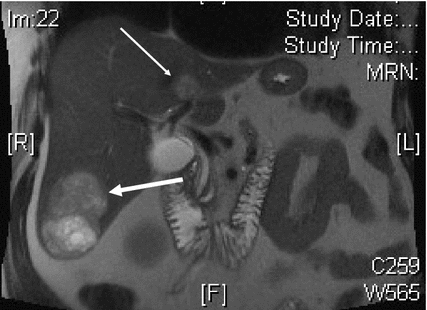
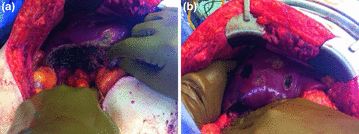

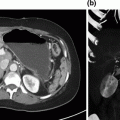
Fig. 2.2
Axial MRI of patient with metastatic NET to liver. Large right segment 6 lesion has grown significantly over 6-month interval (large arrow). Caudate lesion is second largest tumor (small arrow). At laparotomy, attempts were made to remove this lesion, but concerns over damaging left portal triad resulted in radiofrequency ablation of this tumor

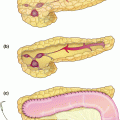
Fig. 2.3
Post-treatment photographs of patient with mid-gut NET. Panel a shows resection of 8 cm segment 6 lesion with hepatic parenchymal preservation. Panel b shows treatment of smaller lesions by enucleation (dark areas) or surface ablation (light areas)
Postoperatively, the patient recovered with no significant change in hepatic functions postoperatively and was discharged on postoperative day 6 without complication. His pathology showed his small bowel lesion was a grade 1, 2.3 cm mass, solitary mid-gut primary with a Ki-67 of <1%. There were 4/22 lymph nodes with metastatic disease. All margins were negative. His large segment 6 lesion which at presentation had been 6 cm, was measured at 8.8 cm and had a Ki-67 of 9.7% with positive tumor margin as expected. There were five left liver nodules between 4 and 6 mm with Ki-67 of zero with no mitoses identified. There were 12 resected right hepatic nodules between 5 and 30 mm and had zero mitoses identified in these lesions.
This patient illustrates several aspects relevant to debulking of neuroendocrine lesions from a mid-gut primary. First, the patient had negative cross-sectional imaging and negative endoscopy in terms of the primary tumor in the terminal ileum and was labeled as an unknown or occult primary with extensive hepatic metastases. He had symptoms related to serotonin production and had an elevated 5HIAA. He had marked elevation of his serum serotonin, essentially confirming a mid-gut primary despite the negative imaging with colonoscopy, capsule endoscopy, and cross-sectional assessment of the bowel. Of note, his postoperative serotonin had dropped to near normal levels at 230 ng/mL. His primary lesion measured 2.3 cm in size despite the negative imaging, negative octreoscan, and negative capsule endoscopy. It was able to be palpated very easily on exposure of the bowel. This is very typical, and extensive efforts sometimes are costly to identify the primary and are not necessary with this clinical scenario. Also patients may sometimes be discouraged from undergoing surgical debulking of the hepatic metastases because of the occult primary and this should not be a hindrance, as again it can virtually always be identified by surgical exploration and treated simultaneously with debulking liver metastases.
The initial strategy employed for this patient was a watch and wait with Sandostatin and although the majority of the lesions in his liver remain stable, he had a solitary lesion increase from 6 to 8 cm that then was 8.8 cm at the time of resection. This is somewhat unusual, but prompted a referral to a tertiary center which led to this successful debulking. The final pathology demonstrated that specific lesion had an intermediate and almost high grade Ki-67 of 9.7%, whereas both the primary lesion and the other liver metastases had Ki-67 of <1%. Clearly a secondary mutation had occurred in this specific lesion, leading to its rapid growth, and also demonstrating the importance of removing that before it became too large or it had metastasized outside the liver. This patient has been followed for over 12 months with no clear new lesions appearing, with complete resolution of the symptoms, and no postoperative complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree