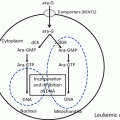
Fig. 4.1
Chemical structures of ABL tyrosine kinases. a Imatinib mesylate, b dasatinib, c nilotinib, d bosutinib, e bafetinib, f ponatinib
Point mutations within the ABL kinase domain, which interfere with imatinib binding, are the most critical cause of imatinib resistance [5, 6]. To overcome this, four second-generation ATP-competing ABL TKIs, dasatinib [7], nilotinib [8], bosutinib [9], and bafetinib [10], have been developed. Studies show that dasatinib and nilotinib are more effective than imatinib when used to treat naive CML patients in the chronic phase (CP) of the disease [11, 12]. Despite promising clinical results, a common mutation, T315I, is not effectively targeted by any of the second-generation ABL TKIs [13]. Therefore, a third-generation ABL TKI, ponatinib, was developed and shows good clinical efficacy against CML cells harboring the T315I mutation [14]. Thus, treatments for CML are progressing rapidly, and further evolution is expected.
TKIs are classified as Type I (imatinib, nilotinib, bafetinib, and ponatinib), which completely occupy the ATP-binding pocket, and Type II (dasatinib and bosutinib), which partially occupy the ATP-binding pocket (Fig. 4.2) [15]. Type I ABL TKIs bind to the kinase domain of ABL only when the domain adopts an inactive or “closed” conformation. Conversely, Type II ABL TKIs bind to the kinase domain of ABL when the domain adopts the active or “open” conformation [16]. For this reason, Type II ABL TKIs are generally more potent, but less specific, than Type I ABL TKIs.
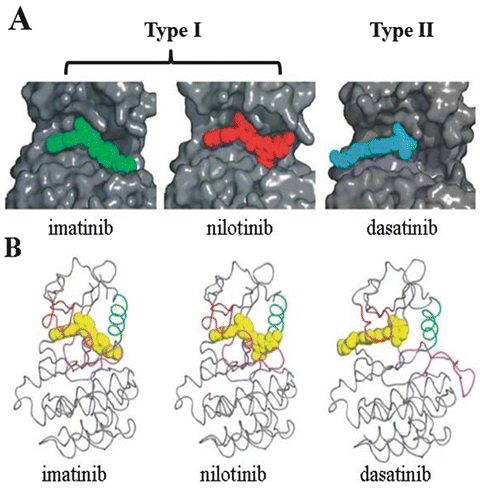

Fig. 4.2
Structure of ABL complexed with imatinib, nilotinib, and dasatinib. a Crystal structures of ABL kinase complexed with imatinib (green), nilotinib (red), and dasatinib (blue). Residues from the nucleotide-binding loop (P-loop) and activation loop (A-loop) are omitted from the surface calculation to improve clarity. b Comparison of the different binding modes of imatinib (left), nilotinib (middle), and dasatinib (right). The positions of the P-loop (red) and A-loop (magenta) vary according to whether the kinase is in an active conformation [in which the P-loop adopts an extended conformation and the N-terminal end of the activation loop adopts a “DFG-in” conformation (right)] or an inactive conformation [in which the P-loop is bent over the inhibitor and the N-terminal end of the activation loop adopts a “DFG-out” conformation (left and middle)]. Imatinib and nilotinib block the kinase in an inactive conformation. The green helix is helix C, which often moves between the active and inactive states (Adapted and modified from 15)
4.2 Second-Generation ABL TKIs
4.2.1 Dasatinib (Sprycel®)
4.2.1.1 Structure and Preclinical Studies
The chemical formula of dasatinib (Fig. 4.1b) is C22H26ClN7O2S, and its molecular weight is 488 g/mol. The International Union of Pure and Applied Chemistry (IUPAC) name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide. Dasatinib was developed as a SRC family kinase (SFK) inhibitor. Its affinity for the ABL kinase domain is 325-fold higher than that of imatinib; this was achieved by modifying the piperazinyl ethanol based on thiazole [7]. Imatinib forms six hydrogen bonds with the ATP-binding pocket, while dasatinib forms only two. Also, dasatinib inhibits more than 50 tyrosine kinases, including BCR-ABL, all nine SFKs (SRC, LCK, LYN, FGR, YES, FYN, HCK, BLK, and FRK), c-KIT, EPHA2, and PDGFR-β, suggesting that its specificity is relatively low. Although dasatinib is effective against most mutated BCR-ABLs, its effect on BCR-ABL harboring T315I/A, F317 L/V, V299 L, Q252H, and E255K/V mutations is limited (Fig. 4.3) [7, 17, 18].
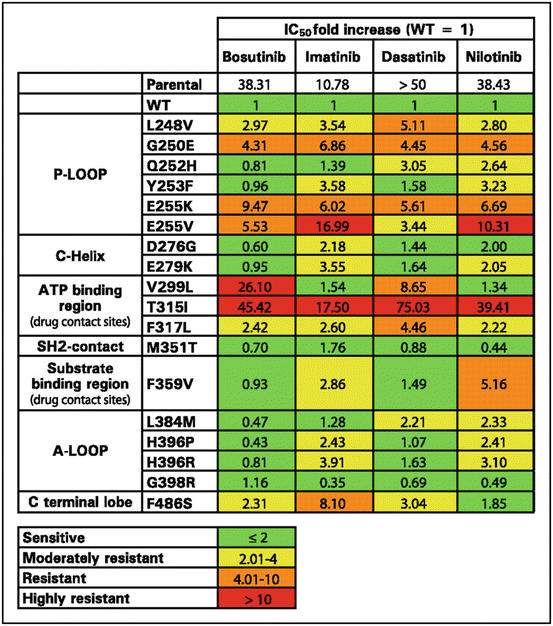

Fig. 4.3
IC50 values for bosutinib, imatinib, dasatinib, and nilotinib against 18 mutated forms of BCR/ABL expressed in Ba/F3-transfected cells. IC50, relative concentration that inhibits 50%; WT wild type, P-loop phosphate-binding loop, ATP adenosine triphosphate, SH2 Src homology 2, A-loop activation loop (Adapted from 18)
4.2.1.2 Pharmacokinetics
The T max (the time at which the maximum serum concentration, C max, is observed) of dasatinib is estimated to be approximately 0.5 h. Both dasatinib and the total radioactivity plasma concentration decrease rapidly, with an elimination half-life (T 1/2) of less than 4 h [19]. Because the T 1/2 of dasatinib is very short, the initial recommendation is that it be administered in twice-daily (bis in die; BID) doses. However, a once-daily (quaque die; QD) dose of 100 mg is as effective as 70 mg BID and has fewer adverse effects [20]. Thus, dasatinib is usually administered to adults with chronic phase CML (CML-CP) at a dose of 100 mg QD. This suggests that inhibiting ABL kinase for only several hours per day is enough to prevent CML progression and that constitutive suppression of multiple kinases might induce a greater number of adverse effects [20]. We also showed that a 2 h exposure of CML cells to another second-generation ABL TKI, bafetinib, was enough to inhibit proliferation [21].
With respect to its interaction with other agents, a previous study showed that dose-adjusted area under the concentration-time curve (AUC) for dasatinib over the first 4 h of a 12 h dosage interval (AUC[0–4]) in patients taking an H2 receptor antagonist or proton pomp inhibitor (PPI) was significantly lower than that in patients not taking an acid suppressant [22]. Also, CYP3A4 inhibitors may increase the levels of dasatinib in the system. Thus, clinicians should consider reducing the dose of dasatinib if a patient is also taking CYP3A4 inhibitors. By contrast, CYP3A4 inducers may reduce the systemic levels of dasatinib. Thus, it may be necessary to increase the dose when CYP3A4 inducers are used simultaneously [23].
4.2.1.3 Clinical Effects
A phase 2 clinical trial in which 387 imatinib-resistant/intolerant patients with CML-CP received 70 mg of dasatinib BID (140 mg/day) was performed, and a complete cytogenetic response [CCyR: a state in which the Philadelphia (Ph) chromosome was not detected] was achieved in 49% of cases [24].
Another clinical trial [the dasatinib versus imatinib study in treatment-naive CML patients (DASISION) trial] examined the use of first-line dasatinib immediately after a diagnosis of CML. Dasatinib (100 mg QD) or imatinib (400 mg QD) was administered to previously untreated patients with CML-CP: a CCyR was observed in 77% and 66% of cases, respectively, after 12 months. Also, a major molecular response [MMR: a ≥3 log reduction in BCR-ABL mRNA levels as measured by real-time quantitative PCR (RQ-PCR)] was observed in 46% and 28% of cases, respectively. The time to MMR was significantly shorter in the dasatinib group (Table 4.1a) [11].
Table 4.1
Clinical effects of first-line dasatinib and nilotinib
a. Dassision (dasatinib) | b. ENESTnd (nilotinib) | |||||||
|---|---|---|---|---|---|---|---|---|
Imatinib | Dasatinib | Imatinib | Nilotinib | Nilotinib | ||||
400 mg QD | 100 mg QD | 400 mg QD | 300 mg BID | 400 mg BID | ||||
CCyR | CCyR | |||||||
3 M | 31% | 54% | ||||||
6 M | 59% | 73% | 6 M | 45% | 67% | 63% | ||
9 M | 67% | 78% | ||||||
12 M | 72% | 83% | 12 M | 65% | 80% | 78% | ||
MMR | MMR | |||||||
3 M | 0.40% | 8% | 3 M | 1.00% | 9% | 5% | ||
6 M | 8% | 27% | 6 M | 12% | 33% | 30% | ||
9 M | 18% | 39% | 9 M | 18% | 43% | 38% | ||
12 M | 28% | 46% | 12 M | 22% | 44% | 43% | ||
Progression to AP/BP | 3.50% | 1.90% | Progres-sion to AP/BP | 4% | <1% | <1% | ||
Dasatinib is indicated not only for CML but also for Ph chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Patients with newly diagnosed Ph+ALL received dasatinib induction therapy for 84 days, which is combined with steroids for the first 32 days and intrathecal chemotherapy. At 20 months, overall survival was 69.2%, and disease-free survival was 51.1%. In adults with Ph+ALL, induction treatment with dasatinib plus steroids leads to a complete hematologic response (CHR) in virtually all patients irrespective of age, with good compliance, no deaths, and a rapid debulking of the neoplastic clone [25]. When 34 patients with relapsed Ph+ALL or CML-lymphoid blast crisis (CML-LB) were treated with a combination of dasatinib and hyper-CVAD, 84% achieved CCyR after one cycle of therapy. Overall, 42% of patients achieved a complete molecular response (CMR: defined as bcr-abl mRNA negativity as measured by RQ-PCR), and 35% had a MMR [26].
Imatinib does not prevent central nervous system (CNS) relapse due to poor penetration through the blood-brain barrier [27]. Although dasatinib is a substrate of P glycoprotein (as is imatinib) [28], the high potency of dasatinib means that residual concentrations in the CNS are sufficient to prevent the proliferation of CML cells. Thus, dasatinib has therapeutic potential for the management of intracranial leukemic disease and shows substantial clinical activity in patients who experience CNS relapse while receiving imatinib therapy [29].
4.2.1.4 Adverse Effects
Pleural effusion and thrombocytopenia are observed slightly more frequently, and edema, gastrointestinal symptoms, muscle-related symptoms, and rash are observed less frequently, with dasatinib than with imatinib [11]. Dasatinib-induced platelet dysfunction can cause clinically significant bleeding [30]. Adverse effects such as colitis and pleuritis are common and are preceded by large granular lymphocyte (LGL) lymphocytosis [31]. Recent reports of pulmonary arterial hypertension in those taking dasatinib have raised concerns about the long-term sequelae of drugs that may need to be administered for decades [32].
4.2.1.5 Indications and Usage
Dasatinib is indicated for the treatment of adult patients with newly diagnosed CML-CP, for adults with all phases of CML[-CP/accelerated (AP)/blastic phase (BP)] showing resistance or intolerance to prior therapy (including imatinib), and for adults with Ph+ALL showing resistance or intolerance to prior therapy. A dose of 100 mg QD is recommended for CML-CP patients, whereas 140 mg QD is recommended for patients with CML-AP/BP or Ph+ALL. Dasatinib is administered orally, either with or without food.
4.2.1.6 Immunoeffects
A subset of patients treated with dasatinib show an increased number of LGL, and the outcome is reported to be more favorable in such patients [33]. Generally, the greater the dose of dasatinib, the higher the incidence of LGL lymphocytosis. This is thought to be due to direct stimulation of LGL proliferation or to inhibition of LGL-suppressing regulatory T cells (Tregs) by dasatinib [34]; however, the mechanistic details are unclear.
Dasatinib induces a rapid, dose-dependent, and substantial mobilization of non-leukemic lymphocytes and monocytes in the blood, peaking 1–2 h after oral intake; the blood counts closely mirror the plasma concentration of the drug. A previous study showed preferential mobilization of natural killer (NK), NKT, B, and γδ+ T cells, coupled with more effective transmigration of leukocytes through an endothelial cell layer, along with improved NK cell cytotoxicity [35].
4.2.1.7 Stop Studies
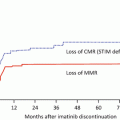
Only one dasatinib stop study [the dasatinib discontinue (DADI) trial] has been published [36]. Dasatinib treatment subsequent to imatinib was discontinued after confirmation of a stable deep molecular response (DMR; defined as bcr-abl mRNA negativity as measured by RQ-PCR) for more than 1 year. At a median follow-up of 20 months after discontinuation, the estimated treatment-free remission (TFR) rates were 49% at 6 months and 48% at 12 months (Fig. 4.4a). The TFR rate at 12 months was significantly poorer in imatinib-resistant patients (8%) than in other patients (58%) (p = 0.0001) (Fig. 4.4b). The high NK cell and low γδ+ T cell and CD4+ Tregs (CD25+CD127low) counts observed before discontinuation were significantly correlated with successful discontinuation of therapy. All molecularly relapsed patients returned to DMR within 6 months after the reintroduction of dasatinib. These findings suggested that discontinuation of dasatinib after sustained DMR for more than 1 year is feasible, particularly in patients without a prior history of imatinib resistance.
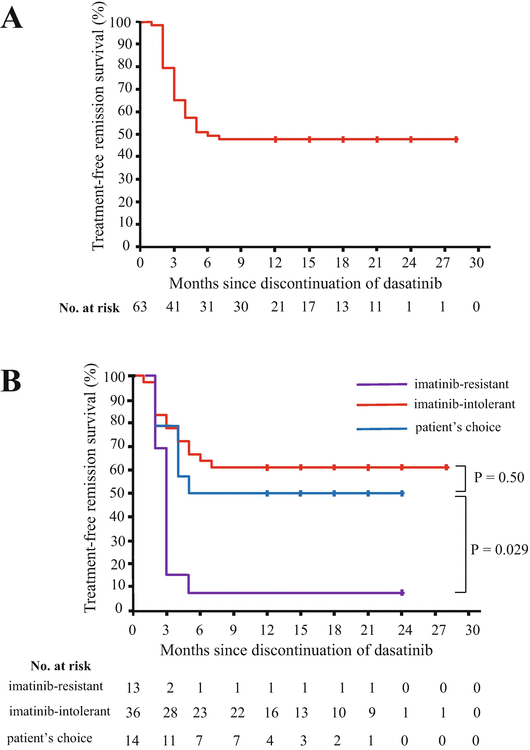

Fig. 4.4
Kaplan-Meier estimates of treatment-free survival in patients with chronic myeloid leukemia after discontinuation of dasatinib. a For all 63 patients, the estimated treatment-free remission rate was 49% (95% CI, 36–61%) at 6 months and 48% (95% CI, 35–59%) at 12 months. b Treatment-free remission rates based on the reasons for switching patients from imatinib to dasatinib (Adapted from 36)
4.2.2 Nilotinib (Tasigna®)
4.2.2.1 Structure and Preclinical Studies
The chemical formula of nilotinib (Fig. 4.1c) is C28H22F3N7O, and its molecular weight is 530 g/mol. The IUPAC name is 4-methyl-N-[3-(4-methyl-1H-imidazol -1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide. Structural modification of imatinib (to generate nilotinib) led to a 30-fold increase in anti-CML activity. Nilotinib forms four hydrogen bonds with the ATP-binding pocket, and it suppresses ABL, PDGFR, and c-KIT activity, but not SFK activity. The affinity of imatinib for PDGFR and c-KIT is stronger than that for ABL, whereas the affinity of nilotinib for ABL is stronger than that for other kinases. Thus, nilotinib has higher specificity for ABL than imatinib and other TKIs, including dasatinib and bosutinib [8]. Although nilotinib is effective against most forms of mutated ABL (except T315I), its effects against ABL harboring E255K/V, Y253F/H, Q252H, and F359 V are limited (Fig. 4.3) [8, 17, 18].
4.2.2.2 Pharmacokinetics
The T max of nilotinib is 3 h, and the T 1/2 following multiple daily dosing is approximately 17 h, which is much longer than that of dasatinib. One important issue with nilotinib is that the type and quantity of food affect its absorption. When administered after a high-fat meal, the AUC of nilotinib in CML patients increases by 50% [37].
Nilotinib is a competitive inhibitor of cytochromes CYP3A4, CYP2C8, CYP2C9, and CYP2D6 [38]. Single-dose or repeated-dose administration of nilotinib results in weak and moderate inhibition of CYP3A, respectively [39]. Thus, care must be taken when it is administered in combination with other agents that affect CYPs. In addition, PPIs cause a modest reduction in the rate and extent of nilotinib absorption [40]. With respect to transporters, OCT-1-mediated influx may be a key determinant of the molecular response to imatinib; however, it is unlikely to impact cellular uptake and patient responses [41].
4.2.2.3 Clinical Effects
In a phase 2 open-label study, 400 mg of nilotinib was administered BID to patients with CML-CP after imatinib failure or intolerance. At 6 months, the rate of major cytogenetic response (McyR, Ph ≤35%) was 48% and that of CCyR was 31%. Nilotinib was effective in patients harboring BCR-ABL mutations associated with imatinib resistance (except T315I) and also in patients with a resistance mechanism that was independent of BCR-ABL mutations [42].
Another study reviewed the outcome of 420 patients with CML post-imatinib failure (resistance-recurrence in 374; toxicity in 46). The estimated 3 year survival rates were 72% in 88 patients who progressed to the chronic phase, 30% in 130 patients who progressed to the AP, 7% in 156 patients who progressed to the BP, and 75% in 37 CP patients who were imatinib intolerant [43].
The Efficacy and Safety in Clinical Trials – Newly Diagnosed Patients (ENESTnd) study examined patients with previously untreated CML-CP. Patients received nilotinib at 300 or 400 mg BID or imatinib at 400 mg QD. After 12 months, the MMR rate was 44% in the nilotinib 300 mg BID group, 43% in the nilotinib 400 mg BID group, and 22% in the imatinib group (Table 4.1b) [12].
Another study reported that, after a priming procedure with peginterferon α-2a (90 μg per week for 1 month), CML patients received peginterferon α-2a (45 μg per week) combined with nilotinib (600 mg daily) up until 24 months after interferon initiation. At 12 months, 17% of patients had achieved more than 4.5 log reduction of BCR-ABL mRNA determined RQ-PCR (MR4.5). Despite substantial toxicity, most patients remained on the study drugs for more than 1 year [44].
Imatinib and dasatinib have been registered for the treatment of Ph+ALL. Nilotinib has also been tested as a second-line drug and can offer an alternative to patients who fail to respond to a first-line drug [45]. The combination of nilotinib and high-dose cytotoxic drugs is feasible, achieving a high cumulative CMR and hematologic relapse-free survival rates [46].
4.2.2.4 Adverse Effects
In a phase 2 trial involving imatinib-resistant/imatinib-intolerant CML-CP patients, grade 3 or higher side effects, including thrombocytopenia, neutropenia, and increased levels of bilirubin and lipase, were observed. Cross-intolerance with imatinib was rare [42]. The ENESTnd study revealed no significant difference between imatinib and nilotinib in terms of safety. Rash, headache, and mild increases in liver and pancreatic enzyme levels were observed slightly more frequently in patients receiving nilotinib, but edema, muscle convulsions, and neutropenia were observed less frequently [12].
Recent reports show that nilotinib may be associated with an increased risk of vascular adverse events, including peripheral artery occlusive disease (PAOD) [47, 48]. Despite this, the risk of developing PAOD while on TKI therapy is unclear, and causality has not been established. A retrospective cohort analysis examined the rates of PAOD in CML-CP patients treated with imatinib or nilotinib or with non-tyrosine kinase-based therapy. Nilotinib was associated with higher rates of PAOD than imatinib [49]. Although the underlying mechanisms remain unknown, patients should be screened for PAOD and other vascular risk factors such as diabetes mellitus prior to starting nilotinib and again during therapy.
4.2.2.5 Indication and Usage
Nilotinib is indicated for the treatment of adult patients with newly diagnosed CML-CP and for the treatment of CML-CP/AP in adults resistant or intolerant to prior therapies, including imatinib. Nilotinib should be taken BID at approximately 12 h intervals and on an empty stomach. No food should be consumed for at least 2 h before and for at least 1 h after the drug is taken. Newly diagnosed CML-CP patients should receive 300 mg BID nilotinib, and those with resistant or intolerant CML-CP/AP should receive 400 mg BID.
4.2.2.6 Immunoeffects
Nilotinib inhibits the proliferation and suppressive capacity of Tregs in a dose-dependent manner. However, the production of cytokines by Tregs and CD4+CD25− T cells is only inhibited at high concentrations of nilotinib (i.e., doses exceeding the mean therapeutic serum concentration of the drug). Nilotinib does not hamper Tregs function at clinically relevant doses [50].
4.2.3 Bosutinib (Bosulif®)
4.2.3.1 Structure and Preclinical Studies
The chemical formula of bosutinib (Fig. 4.1d) is C26H29Cl2N5O3, and its molecular weight is 530 g/mol. The IUPAC name is 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy] quinolone-3-carbonitrile. The affinity of bosutinib for ABL is about 50-fold higher than that of imatinib, and it shows characteristically weak inhibitory activity against c-KIT and PDGFR, which can sometimes cause adverse effects in patients treated with imatinib. The affinity of bosutinib for ABL is weaker than that of dasatinib, while that of bosutinib for SFKs (including SRC, LYN, HCK, and FYN) is almost ten times higher than that of dasatinib. However, bosutinib has only a very weak inhibitory effect on other SFKs, including YES, LCK, FGR, and BLK [9]. A conserved water-mediated hydrogen bond network defines the kinase selectivity of bosutinib [54].
4.2.3.2 Pharmacokinetics
A study showed that treatment with single and multiple doses of bosutinib resulted in slow absorption, with a median time to T max of 4–6 h and a T 1/2 from 13 to 22 h [56]. Preliminary assessments showed that the C max increased by ~2.52-fold and the AUC by ~2.28-fold when bosutinib (200 mg) was administered with food rather than under fasting conditions; administration of 400 mg of bosutinib with food increased the AUC by ~1.5-fold. Under fed conditions, bosutinib exposure was linear, and the dose was proportional, and the C max increased by ~1.5-fold. The T 1/2 supports a once-daily dosing regimen [57].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree