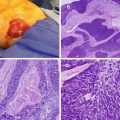
Fig. 21.1
Photograph depicting tumor implants from pseudomyxoma peritonei nearly replacing the omentum, a finding referred to as “omental cake,” from a ruptured appendiceal neoplasm
Regional approaches to peritoneal surface disease have included cytoreduction via peritonectomy procedures [8, 9], intraperitoneal injection of a streptococcal preparation OK432 [10], debulking with photodynamic therapy [11–13], intracavitary immunotherapy [14], and early postoperative intraperitoneal chemotherapy [2, 15–17]. In fact, intraperitoneal chemotherapy has also been studied preceding and following cytoreduction [18]. Delivery at the time of cytoreduction has been thought to have a more complete distribution throughout the peritoneal cavity given that adhesions may be present before and after surgery. Intraperitoneal chemotherapy has achieved higher concentrations at the level of the peritoneal surfaces than systemic chemotherapy before reaching toxic endpoints, and the addition of heat has been thought to have a synergistic effect with the chemotherapy [19]. Thus, the advantage of CRS-HIPEC is that it addresses macroscopic diseases surgically and microscopic diseases chemically and is now routinely performed in specialized centers across America, Europe, Asia, and Australia [3]. Although hyperthermic chemotherapy has carried many names during its development including continuous hyperthermic peritoneal perfusion (CHPP), hyperthermic antiblastic peritoneal perfusion (HAPP), heated intraoperative intraperitoneal chemotherapy (HIIC), intraperitoneal hyperthermic chemotherapy (IPHC), and intraperitoneal hyperthermic perfusion (IPHP), “HIPEC” is now the preferred acronym by international consensus [2, 20].
Decision Process
Once a diagnosis of peritoneal surface disease has been made and the patient wishes to pursue treatment, the clinician should ask the following important question: is this patient an appropriate candidate for CRS-HIPEC? The answer to the above question requires a thorough assessment of the disease process, the patient’s performance status, and an in depth understanding of the clinical utility of CRS-HIPEC.
Goal of CRS-HIPEC
Complete cytoreduction of all gross diseases is the primary objective of CRS-HIPEC as complete cytoreduction is a strong predictor of improved survival [14, 21, 22]. To this end, many guidelines for selecting appropriate situations for CRS-HIPEC are aimed at identifying cases in which complete cytoreduction is feasible. While cure can be achieved in a minority of cases, it is important to realize that most patients will recur despite a complete resection. Attempts are made to reduce as much of the tumor burden as possible without compromising the safety of the patient for cases in which complete cytoreduction is not feasible [14].
Evaluation of Patients with PSD for Possible CRS-HIPEC
Indications
The classic indication for CRS-HIPEC is PSD from low-grade appendiceal neoplasms otherwise known as pseudomyxoma peritonei (PMP) [14]. Other generally accepted indications include PSD arising from appendiceal adenocarcinoma, colorectal cancer, gastric cancer, ovarian cancer, and peritoneal mesothelioma [19]. This therapy has also been applied to small bowel adenocarcinoma [23, 24], appendiceal adenocarcinoid [19], sarcomatosis [25–30], endometrial cancer [31], and urachal cancer [32]. Although CRS-HIPEC has been offered to patients with PSD from biliary or pancreatic primary tumors, these are generally avoided due to difficulty in controlling the primary tumor [14].
In general, lower-grade primary tumors have better outcomes with CRS-HIPEC than higher-grade lesions given the lower rate of locoregional recurrence. The rate of recurrence in high-grade tumors is attributable to the early invasion of peritoneal surfaces and subsequent smaller margins of excision obtained with peritonectomy procedures [19].
Patient Selection
It is imperative that appropriate candidates be selected for CRS-HIPEC due to the considerable morbidity associated with this therapy. A determination of the appropriateness of CRS-HIPEC in a given patient is based upon a thorough clinical assessment and extensive imaging [33, 34]. Preoperative evaluation generally includes complete history, physical examination, pathologic review, contrast-enhanced computed tomography (CT), and laboratory examination including blood counts, electrolytes, and liver function panel. Previously published selection criteria include:
Besides being largely subjective, selection criteria are quite variable. A patient may be considered medically unfit for CRS-HIPEC if they display signs of organ dysfunction evidenced by elevations in serum creatinine or liver enzymes or abnormally low white blood cell or platelet counts [14]. Although not an absolute contraindication, poor performance status has predicted worse outcomes for patients presenting for consideration for CRS-HIPEC. Patients with Eastern Cooperative Oncology Group (ECOG) performance status scores of 2 or greater have significantly worse survival outcomes than those with better scores [36].
1.
The patient is sufficiently medically fit to undergo CRS-HIPEC.
2.
There is no extra-abdominal disease.
3.
Peritoneal disease burden is potentially completely resectable or at least significantly reducible.
4.
There are no parenchymal hepatic metastasis.
The extent of previous surgery has been shown to correlate with the extent of tumor implants throughout the peritoneal cavity. Sugarbaker and Chang created a prior surgical score (Table 21.1) and found that patients with scores of 3 had significantly worse survival when compared with patients with lesser scores [37].
Table 21.1
Prior surgical score
Prior surgical score | Description |
|---|---|
0 | PSD diagnosed via biopsy or laparoscopy |
1 | Previous laparotomy without resection |
2 | Previous laparotomy with resection |
3 | Previous attempt at complete cytoreduction |
Other clinical factors are able to predict outcome and can be used to identify the most appropriate candidates for CRS-HIPEC. The presence of a bowel obstruction with subsequent malnutrition has also predicted worse survival in this population of patients [35]. Data from our own institution indicates that malignant ascites is a strong predictor of incomplete resection, and as such also predicts worse survival [38].
Preoperative imaging plays an important role in determining the location and extent of disease. Specifically, radiographic studies are used to exclude extra-abdominal disease, parenchymal hepatic metastases, extensive small bowel involvement, and obstruction of the small bowel, ureters, or biliary tree [14]. Contrast-enhanced CT of the chest, abdomen, and pelvis is the standard preoperative imaging modality (Fig. 21.2). Sensitivity for detecting peritoneal lesions ranges from 25 to 37 % and improves with increasing lesion size [32, 37]. For example, lesions less than 5 mm in thickness were only detected in 28 % of cases [39]. Although the sensitivity for detecting peritoneal lesions is low, this imaging modality is good for determining retroperitoneal solid organ involvement and overall operability [35]. Furthermore, patients without mesenteric tumors greater than 5 mm and small bowel obstruction have more than a 90 % chance of having a complete resection [34, 40]. Magnetic resonance imaging (MRI) with both oral and intravenous contrast is able to detect PSD with 84–100 % sensitivity [41, 42]. Unfortunately, MRI is also associated with a significant rate of false positives as it is incapable of distinguishing between postoperative scar formation and PSD [19]. Positron-emission tomography (PET) is good both for high-volume disease and for ruling out extra-abdominal disease, yet sensitivity and specificity decrease to 10 % and 42 %, respectively, in patients with low-volume disease [35, 43, 44].
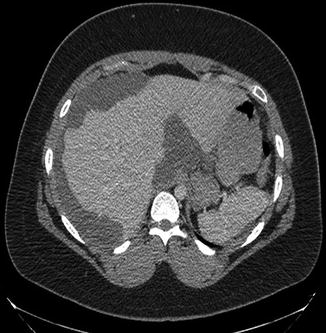

Fig. 21.2
Contrast-enhanced computed tomography of a patient with large-volume pseudomyxoma peritonei. Tumor encases the lateral liver and porta hepatis. A smaller amount of disease is deposited on the spleen
Despite the shortcomings for PSD, modern imaging is able to provide valuable information during the preoperative patient evaluation. Some centers will offer CRS-HIPEC to patients with parenchymal hepatic metastasis if they are amenable to resection at the time of surgery [43]. Others have specific criteria for offering CRS-HIPEC to patients with PSD from different primary tumors based on estimations of disease volume [21]. In the case of PSD from ovarian cancer, no contraindications regarding tumor metastases have been agreed upon [45]. Extensive disease in the lesser sac has been shown to decrease the likelihood of complete cytoreduction and can be identified on cross-sectional imaging when this potential space is distended in the setting of known carcinomatosis [46].
Beyond radiographic imaging, other components of a thorough preoperative evaluation may be case specific. Endoscopy with or without endoscopic ultrasonography can provide valuable information for patients with gastric or colorectal primary tumors [21]. Diagnostic laparoscopy has also been employed to determine the resectability of PSD prior to CRS-HIPEC [47, 48].
Staging Peritoneal Surface Disease
Although the majority of staging will occur at cytoreduction, the use of diagnostic laparoscopy makes it possible to stage patients prior to CRS-HIPEC. The two most commonly used staging systems for PSD are the Gilly carcinomatosis index [49] and the peritoneal carcinomatosis index (PCI) [50]. Both are based upon lesion size and extent of distribution throughout the abdomen. The Gilly carcinomatosis index is the simpler of the two with a designation of Stage 0 to Stage 4 as follows: Stage 0 for no macroscopic disease, Stage 1 for localized tumor implants less than 0.5 cm in diameter, Stage 2 for non-localized tumor implants less than 0.5 cm in diameter, Stage 3 for localized or non-localized implants 0.5–2 cm in diameter, and Stage 4 for any implants greater than 2 cm in diameter. As expected, higher stage correlates with worse prognosis [15, 49, 51, 52]. Despite being more complex, the PCI is the most widely used PSD staging system largely due its prognostic value [35, 41, 53–55]. Furthermore, it has provided a way to standardize volume and extent of disease and is even used as a gauge of when HIPEC is warranted [43]. Calculating the PCI involves dividing the abdomen into nine regions and the small bowel into four regions. For each region, a score of 0 (no tumor), 1 (tumor up to 0.5 cm), 2 (tumor up to 5 cm), or 3 (tumor > 5 cm) is applied. Scores for each of the 12 regions are tabulated to derive the PCI score (Fig. 21.3) [35, 54].
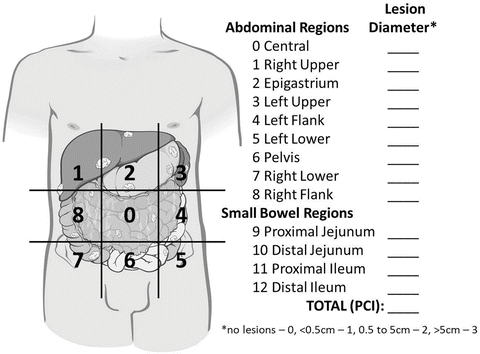

Fig. 21.3
Schematic for calculating the peritoneal carcinomatosis index (PCI) staging system. Scores based on lesion size for each of nine abdominal regions plus 4 small bowel regions are added together to reach the PCI
Management of PSD with CRS-HIPEC
Preoperative Preparation
Once it has been decided that a patient has an acceptable indication for CRS-HIPEC and is an appropriate candidate, he or she is scheduled for surgery. Patients are generally admitted the day prior to surgery for final assessment and preparation. Final assessment includes an interval history, physical examination, and laboratory evaluation consisting of blood counts, comprehensive chemistry panel, appropriate tumor markers, and a blood type with crossmatch of 4 units of packed red blood cells. For patients felt to have a higher likelihood of ureteral involvement based on disease volume or prior surgery, a urology consultation may be obtained for the placement of externalized ureteral stents. A bowel preparation is routine for all patients and may be accomplished with the use of enemas in cases where a bowel obstruction is present.
Cytoreduction
Patients may be placed in a supine or in a modified lithotomy position for CRS-HIPEC. If a modified lithotomy position is used, the surgeon must be cautious about positioning the legs to prevent myonecrosis of the posterior compartment of the legs [8]. After a thorough prep, an incision is made from the xiphoid to the pubis for generous exposure of the peritoneal cavity. If the falciform ligament is present, it is resected prior to placing a fixed retractor. All adhesions from the disease process or previous operations are lysed to facilitate penetration of HIPEC to all areas of the peritoneal cavity. Cytoreductive surgery is then undertaken to remove all gross disease if technically feasible. Peritoneal surfaces with tumor deposits are stripped from the abdominal wall and diaphragm using electrocautery [8, 9, 19]. The greater omentum is routinely removed along with any involved tissue or organ not vital to the patient. If at any time during the procedure, complete cytoreduction is believed to be either not feasible or unsafe for the patient, the remaining tumor is debulked as much as possible. If a bowel resection is required, an anastomosis may be created prior to or following HIPEC. Ostomies are created following HIPEC to allow exposure of the chemotherapy to any potential microscopic disease remaining on the serosal surfaces.
Grading Resection
The degree of resection is judged by the surgeon at the conclusion of the cytoreduction. Residual disease is evaluated by measuring the diameter of the largest remaining tumor deposits. The two predominating classification systems are the R status of resection and the completeness of cytoreduction (CC) score (Table 21.2). Complete cytoreduction of all gross disease is designated with an R status of R0 or R1 or a CC designation of CC-0. The definition of a complete resection is controversial. Some consider a “complete cytoreduction” one in which there is no visible disease at the conclusion of the cytoreductive procedure. Other authors tailor the definition based upon the primary tumor and the expected response to HIPEC. For example, complete cytoreduction for gastric cancer is limited to CC-0, while complete cytoreduction for low-grade appendiceal primary tumors includes CC-0 to CC-1 and CC-0 to CC-2 for ovarian cancer [1, 19].
Table 21.2
Grading resection
Size of residual disease (cm) | R status | CC score [19] |
|---|---|---|
0 | – R0 – negative margins on final pathology R1 – positive margins on final pathology | CC-0N – no visible disease following neoadjuvant chemotherapy CC-0S – no visible disease following cytoreduction |
0.25 | R2a | CC-1 |
0.5 | CC-2 | |
>0.5–2 | R2b | |
>2–2.5 | R2c | |
>2.5 | CC-3 |
HIPEC
The decision to perfuse the patient with HIPEC is based primarily on the degree of resection achieved but is also influenced by the institution and primary tumor. Sugarbaker [19] has described 11 well-defined factors influencing the success rates of HIPEC:
1.
Dose of chemotherapeutic agent
2.
Timing of delivery in relation to surgery
3.
Distribution within the peritoneal cavity
4.
Temperature of the tumor implants during perfusion
5.
Size of the tumor implant
6.
Mucinous versus solid tumor morphology
7.
Tumor response to chemotherapy
8.
Number of cycles of chemotherapy
9.
Peritoneal to plasma drug concentration ratio
10.
Synergistic effect with systemic chemotherapy
11.
Extent of previous surgery
While HIPEC is ideal for patients following a complete cytoreduction, it may be beneficial in selected patients following incomplete cytoreduction [19]. Perfusion following R2a or CC-1 resection is routine given that locally administered chemotherapy is expected to penetrate tumors at depths ranging from 0.1 cm up to 0.25 cm [19, 33]. Thus, tumor deposits up to 0.5 cm in diameter can be effectively treated with chemotherapy penetration of 0.25 cm from either side.
The advantage of administering chemotherapy directly into the peritoneal cavity is that higher drug concentrations can be achieved at the tumor interface with decreased systemic absorption and toxicity [21]. Peritoneal to plasma drug concentration ratios vary depending on the molecular weight and water solubility of the agent used and may vary between 20:1 and 2,000:1 (Table 21.3) [19, 35, 56–63]. Selection of a particular agent is largely determined by the primary tumor or response to previous systemic chemotherapy.
Table 21.3
Comparison of chemotherapeutic agents used in HIPEC
Agent | Molecular weight (Da) | Peritoneal fluid to plasma concentration ratio | Applications |
|---|---|---|---|
5-Fluorouracil | 130 | 300:1 [63] | PMP, gastric |
Cisplatin | 300 | 20:1 [57] | PMP, ovarian, mesothelioma, sarcoma |
Doxorubicin | 544 | 975:1 [62] | Ovarian, sarcoma |
Floxuridine | 246 | 2,000:1 [59] | PMP, CRC, gastric |
Mitomycin C | 334 | 75:1 [60] | PMP, CRC, gastric |
Oxaliplatin | 397 | 25:1 [58] | CRC, gastric |
Paclitaxel | 808 | 1,000:1 [61] | ovarian |
Although one randomized-controlled trial was unable to demonstrate improved response rate with the addition of hyperthermia [64], heating the chemotherapy is believed to both increase the penetration of agents into tissue and enhance their cytotoxic effects [19, 65, 66]. In vitro studies have also shown that hyperthermia can lead to blunted angiogenesis, increased enzyme denaturation, and apoptosis [67]. The desired temperature of the perfusate in the abdomen ranges from 40 to 42 °C [14, 21], as temperatures greater than 42 °C have been associated with increased morbidity [68].
If perfusion with HIPEC is anticipated, the patient’s core body temperature is cooled using a variety of passive means including lowering room temperature and ceasing to warm intravenous fluids and airway gases [2, 14, 69]. These maneuvers prevent systemic hyperthermia during hyperthermic peritoneal perfusion. The delivery of HIPEC to the patient has evolved into several different modalities (Table 21.4) [2, 70–73]. Each technique utilizes a closed continuous circuit to maintain consistent hyperthermia and temperature probes located at different points throughout the circuit to monitor the temperature. This circuit involves the use of inflow and outflow catheters carrying the perfusate to and from the abdominal cavity respectively, a pump, and a heat exchanger (Fig. 21.4). Once sufficient flow is established, the chemotherapeutic agent is added to the circuit. Although much debate exists regarding the optimal perfusion modality, the majority of experts agree that there is a lack of evidence favoring one modality over another [2].
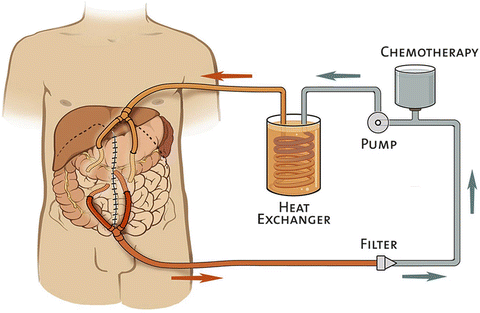
Table 21.4
Comparison of HIPEC modalities
Perfusion modality | Advantages | Disadvantages |
|---|---|---|
Open abdomen (coliseum) | Equal exposure of all surfaces to perfusate | Difficult to maintain high temperature |
Equal temperature distribution | Risk of contamination | |
Easy access to abdominal cavity | Risk of exposure to operating room personnel | |
Closed abdomen | Hyperthermia easily maintained | Unequal exposure of all surfaces to perfusate |
Better tissue penetration | Unequal temperature distribution | |
Minimal risk of exposure to operating room personnel | ||
Peritoneal cavity expander [71] | Equal exposure of intra-abdominal contents to perfusate | Separates abdominal wall from perfusate |
Equal temperature distribution | Risk of exposure to operating room personnel | |
Complex | ||
Abdominal cavity expander [72] | Equal exposure of all surfaces to perfusate | Complex |
Equal temperature distribution | ||
Minimal risk of exposure to operating room personnel | ||
Containment instrument [73] | Equal exposure of all surfaces to perfusate | Complex |
Equal temperature distribution | ||
Easy access to abdominal cavity | ||
Minimal risk of exposure to operating room personnel |

Fig. 21.4
Schematic of hyperthermic intraperitoneal chemotherapy perfusion circuit. (closed technique) (Reprinted from Shen et al. [154]. With permission from Springer Verlag)
One of the most common modalities is the closed abdominal technique (Fig. 21.5). This involves the placement of inflow and outflow catheters through the skin prior to suturing the skin closed in a watertight manner. Temporarily closing at the skin level and leaving the fascia opened allow contact of the perfusate to the abdominal wall. Once flow is established and chemotherapy is added, the abdomen is massaged by the operating room personnel to help distribute the perfusate throughout the abdomen. The increased pressure in the closed technique can facilitate deeper penetration of chemotherapeutic agents into tumor deposits [74, 75].
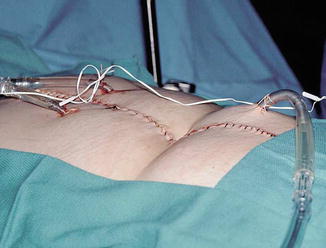

Fig. 21.5
Inflow and outflow catheters in the closed abdominal perfusion technique for hyperthermic intraperitoneal chemotherapy (Reprinted from Stewart et al. [35]. With permission from Springer Verlag)
The open, or coliseum, technique involves suturing a plastic sheet to either side of the patient’s skin incision and to the fixed retractor in effect extending the peritoneal cavity with a “coliseum-like” device. This allows the abdominal contents to float beyond the abdominal wall in a greater volume of perfusate theoretically increasing exposure of all surfaces to the chemotherapy. It also allows the surgeon to manipulate the intra-abdominal contents further facilitating equal distribution of heat and drug throughout the peritoneal cavity. Due to concern regarding exposure of operating room personnel to the chemotherapeutic agent, certain precautions are made including smoke evacuators, education and training of involved personnel, restriction of operating room traffic, filtration masks, and waterproof gowns [76].
Other modalities of perfusion have been developed in an attempt to combine the advantages of both the open and closed techniques. These attempts include the development of a peritoneal cavity expander by a Japanese group [71], an abdominal cavity expander by a French group [72], and an instrument to provide containment of the perfusate by Sugarbaker [73]. While these techniques generally provide equal drug and temperature distribution, they are generally complex and may not eliminate the risk of drug exposure to operating room personnel.
Perfusion is generally maintained for 30–120 min depending on the primary and desired effect. Perfusion times may be decreased to avoid systemic absorption in patients deemed to be particularly susceptible. Factors that may make a patient more susceptible to drug toxicity include extensive peritonectomy, poor performance status, and old age [14]. Once perfusion is completed, the perfusate is drained, and the abdomen re-explored. Ostomies and anastomoses may be made at this time, and drains may be inserted. The abdomen is closed and the procedure is concluded.
Clinical Outcomes for CRS-HIPEC
Complete Cytoreduction
Much effort is devoted to selecting candidates whom will tolerate a complete cytoreduction and thus derive the maximal benefit of CRS-HIPEC [3]. Regardless of the primary tumor, resection status has been shown to be an important independent predictor of survival [14, 81–84]. Although the definition of a complete cytoreduction differs among institutions and primary tumors, the average rate of cytoreduction among high-volume centers is about 75 % [4]. Predictors of complete cytoreduction include good performance status, disease limited to the peritoneal cavity, no more than three resectable hepatic metastases, absence of biliary or ureteral obstruction, absence of more than one intestinal obstruction, absence of small bowel mesenteric implants, and limited disease in the gastrohepatic ligament [43].
Morbidity
Given the extent of the surgical resection required to achieve adequate cytoreduction morbidity is significant. Overall major morbidity following CRS-HIPEC ranges from 12 to 68 % though comparison between studies is difficult due to the lack of a universally accepted grading system [85]. Complications are frequently divided into two groups based on whether they are believed to have arisen from the operation itself or represent toxicity from the chemotherapeutic agent. A multi-institutional review examining the results of over 1,200 procedures found that common complications include reoperation (14 %), neutropenia (13 %), fistula (10 %), pneumonia (9 %), bleeding (8 %), abscess (7 %), sepsis (2 %), bowel obstruction (2 %), and renal insufficiency (1 %) [4]. In addition to bone marrow suppression and renal insufficiency, other observed toxicities attributed to the chemotherapeutic component include transient hepatic toxicity and ileus [27]. More than half of the patients are likely to require a blood transfusion at some point during their operation or hospitalization [36]. Predictors of morbidity include older age, higher PCI, greater number of visceral resections, poorer performance status, and higher drug dose [85, 86]. It is also important to note that morbidity is related to the experience of the center performing CRS-HIPEC as many large centers have displayed a substantial learning curve [85, 87].
Mortality
Operative mortality has been reported as high as 11 % following CRS-HIPEC but is generally about 4 % [35, 85]. Common causes of death are bowel perforation, respiratory failure, bone marrow suppression, thromboembolic events, and various infections. Preoperatively, the presence of ascites, bowel obstruction, and poor performance status predict mortality [35, 36].
Survival by Primary Tumor
Despite the significant rates of morbidity and mortality, CRS-HIPEC remains the only hope many of these patients have for long-term survival. Therefore, any legitimate evaluation of the complications following CRS-HIPEC must be compared to the inherent complications of PSD and its natural history without such treatment. In order to accurately represent expected outcomes, survival is discussed within the context of PSD arising from specific primary tumors.
Pseudomyxoma Peritonei
PMP most commonly results from the peritoneal seeding of ruptured mucinous appendiceal neoplasms and is the classic indication for CRS-HIPEC. PMP has been subdivided based on the relative proportions of mucin and epithelial cells. Disseminated peritoneal adenomucinosis (DPAM) consists primarily of mucin and rare, generally benign-appearing epithelial cells, while peritoneal mucinous carcinomatosis (PMCA) consists of less or no mucin and abundant epithelial cells with cytologic findings consistent with typical carcinomas. PSD with features of both DPAM (abundant mucin) and PMCA (malignant appearing cells) are grouped into a third category [52]. Recent work by our group demonstrated that PMP can be classified as either low grade or high grade with the intermediate cohort being considered low grade except for cases with a signet-ring cell component [88]. Patients with lower-grade lesions or a higher mucin to cell ratio experience better survival [52, 88].
Prior to CRS-HIPEC, patients were subjected to repeated debulking procedures with little hope for long-term survival [22, 33, 89]. Long-term survival following CRS-HIPEC is now common [78, 90–99], but direct comparisons between studies should be made with caution as each contains a different proportion of low- and high-grade lesions, all of which are considered PMP (Table 21.5). A recent multi-institutional review of outcomes following CRS and intraperitoneal chemotherapy (including 2,050 cases where HIPEC was used) reported a median progression-free survival of 98 months and a 10-year overall survival rate of 63 % [84]. Thus, CRS-HIPEC has become the treatment of choice for patients with PMP due to the fact that it has a poor response to systemic chemotherapy and is universally fatal if left untreated [35]. Patients with the lowest-grade lesions amenable to complete resection may expect to do particularly well [33].
Table 21.5
Survival following CRS-HIPEC for pseudomyxoma peritonei
First author | Year | n | Drug | 5-year progression-free survival (%) | 5-year survival (%) | 10-year survival (%) |
|---|---|---|---|---|---|---|
Deraco [93] | 2004 | 33 | MMC + CDDP | 43 | 97 | – |
Stewart [97] | 2006 | 110 | MMC | – | 53 | – |
Yan [99] | 2006 | 50 | MMC | – | 69 | – |
Murphy [96] | 2007 | 83 | MMC | 75 | – | – |
Smeenk [78] | 2007 | 103 | MMC | 37 | 60 | – |
Cioppa [92] | 2008 | 53 | MMC + CDDP | 80 | 94 | 85 |
Elias [94] | 2008 | 105 | Oxaliplatin | 67 | 80 | – |
Baratti [91] | 2009 | 102 | MMC + CDDP | 48 | 84 | 79 |
Vaira [98] | 2009 | 60 | MMC ± CDDP | 80 | 94 | 85 |
Elias [95] | 2010 | 255 | MMC or oxaliplatin | – | 79 | – |
Arjona-Sanchez [90] | 2013 | 38 | MMC | 49 (3 years) | 59 | – |
Colorectal Cancer
A consensus statement on the locoregional treatment of colorectal PSD recommends CRS-HIPEC as the treatment of choice for patients without distant metastatic disease and in whom complete cytoreduction is feasible [43]. This combined therapy has achieved 5-year survival rates in terminally ill patients ranging from 11 to 51 % (Table 21.6) [100–110]. Verwaal and colleagues [102, 107] have undertaken a phase III trial randomizing patients to standard chemotherapy and palliative surgery in cases of obstruction or CRS-HIPEC with a goal of complete cytoreduction prior to HIPEC. They found that disease-specific survival was better in the patients receiving CRS-HIPEC (22.2 months vs. 12.6 months, p = 0.028). Furthermore, 45 % of patients were alive at 5 years following complete macroscopic cytoreductions [102, 107]. Despite these results, HIPEC for this cohort has not been universally accepted in the oncology community, and controversy remains [43, 111].
Table 21.6
Survival following CRS-HIPEC for colorectal cancer
First author | Year | n | Drug | Median OS (months) | 5-year survival (%) |
|---|---|---|---|---|---|
Witkamp [100] | 2001 | 29 | MMC | – | 23 (3 years) |
Pilati [101] | 2003 | 34 | MMC + CDDP | 18 | – |
Glehen [103] | 2004 | 506 | Multiple regimens | 19 | 19 |
Glehen [104] | 2004 | 53 | MMC | 13 | 11 |
da Silva [105] | 2006 | 70 | MMC | 33 | 32 |
Zanon [106] | 2006 | 25 | MMC | 30 | 40 (2 years) |
2008 | 105 | MMC | 22 | 28 (3 years) | |
Shen [110] | 2009 | 197 | MMC | 16 | – |
Elias [108] | 2009 | 48 | Oxaliplatin | 63 | 51 |
Franko [109] | 2010 | 67 | MMC | 35 | 25 |
Gastric Cancer
CRS-HIPEC for peritoneal carcinomatosis arising from gastric cancer has been heavily evaluated and has shown benefits over cytoreduction alone. A randomized control study comparing cytoreduction with HIPEC with cytoreduction without HIPEC observed a median overall survival of 6.5 months for the CRS arm and 11 months for the CRS-HIPEC arm [112]. A systematic review of the literature found that overall median survival for PSD from gastric cancer was 7.9 months, but complete CRS extended survival to 15 months [113]. Typical median survival for these patients following CRS-HIPEC ranges from 6 to 12 months (Table 21.7) [110, 112, 114–120]. Interestingly, HIPEC has also been evaluated in the adjuvant setting in high-risk patients at the time of their initial resection. In one randomized control study, HIPEC delayed recurrence and conferred a survival benefit in patients with either serosal invasion or lymph node metastasis [121].
Table 21.7
Survival following CRS-HIPEC for gastric cancer
First author | Year | n | Drug | Median OS (months) | 5-year survival (%) |
|---|---|---|---|---|---|
Glehen [114] | 2004 | 49 | MMC | 10 | 16 |
Hall [115] | 2004 | 34 | MMC | 8 | 6 |
Yonemura [116] | 2005 | 107 | MMC + CDDP + etoposide | 12 | – |
Scaringi [117]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|