(1)
Institut Bergonié, Bordeaux, France
Abstract
An important group of signalling molecules operate through a pathway which is slightly different from that described in Chap. 1: their receptors do not present tyrosine kinase activity but are coupled to a cytoplasmic tyrosine kinase which ensures, after receptor activation, the transduction of a signal leading to the transcription of target genes. The main signalling pathway downstream the activation of these receptors by their ligands involves cytoplasmic JAK (just another kinase, now Janus kinase) proteins and STAT (signal transducer and activator of transcription) transcription factors, but the activation of other kinases is sometimes possible, with connections with the MAP kinases (Chap. 2) and the PI3 kinase (Chap. 3) pathways.
Whereas the word ‘cytokine’ designates often a large variety of signalling proteins, including growth factors (Chap. 1), TNF-related factors and chemokines (Chap. 6), we will adopt a mechanistic definition and restrict this denomination to the ligands of membrane receptors devoid of tyrosine kinase activity but coupled with cytoplasmic tyrosine kinases. This includes most interleukins and interferons, but excludes many signalling molecules that activate other receptors, such as tyrosine kinase receptors, death receptors, interleukin-1 and toll-like receptors, etc. The cytokines envisaged in this chapter may be involved in multiple cell processes: proliferation and differentiation of blood cells, immunity and inflammation, body weight and size control, antiviral defences, etc.
4.1 Cytokines
About 40 cytokines operating through the activation of a cytoplasmic tyrosine kinase have been identified and can be distributed in two types according to their general structure and that of their receptors (Table 4.1). Type I, characterised by the presence of four α helixes, can be roughly subdivided in several families:
Table 4.1
Main cytokines, cytokine receptors and downstream corresponding effectors
Type | Cytokine | Specific receptor | Family-specific receptor | Kinase | Transcription factor |
|---|---|---|---|---|---|
Type I | IL2 | IL2Rα/β | IL2Rγc (IL2RG) | JAK1 + JAK3 | STAT5 |
IL7 | IL7Rα | ||||
IL9 | IL9Rα | ||||
IL15 | IL15Rα | ||||
IL4 | IL4Rα | STAT6 | |||
IL21 | IL21Rα | STAT1, 3 | |||
GMCSF (CSF2) | GMCSFRα (CSF2RA) | IL3Rβc (CSF2RB) | JAK2 | STAT5 | |
IL3 | IL3Rα | ||||
IL5 | IL5Rα | ||||
IL6 | IL6Rα | gp130 (IL6ST) | JAK1 | STAT1, 3, 5 | |
IL11 | IL11Rα/β | ||||
IL25 | IL17RB | ||||
CNTF | CNTFRα | ||||
LIF | LIFR | ||||
OSM | OSMR | ||||
IL27 (IL30) | IL27Rα | ||||
IL12 | IL12Rβ2 | IL12Rβ1 | JAK2 + TYK2 | STAT4 | |
IL23 | IL23Rα | STAT3 | |||
GH | GHR | JAK2 | STAT5 | ||
PRL | PLR | ||||
OBS (LEP) | OBR (LEPR) | ||||
EPO | EPOR | ||||
TPO (THPO) | TPOR (MPL) | ||||
GCSF (CSF3) | GCSFR (CSF3R) | ||||
Type II | IFNα, β, ω, ε | IFNαR1 | IFNαR2 | JAK1 + TYK2 | STAT1, 2, 3, 5 |
IFNγ | IFNγR1 | IFNγR2 | JAK1 + JAK2 | STAT1, 3, 5 | |
IL10 | IL10Rα | IL10Rβ | JAK1 + TYK2 | ||
IL22 | IL22Rα1/2 | ||||
IL26 | IL20Rα | ||||
IL19, 20 | IL20Rα | IL20Rβ | |||
IL24 | IL22Rα | ||||
Interleukin-2 (IL2) family: IL2, IL4, IL7, IL9, IL15 and IL21.
Interleukin-6 (IL6) family: IL6, IL11, IL27, LIF (leukaemia inhibiting factor), CNTF (ciliary neurotrophic factor) and OSM (oncostatin M).
Interleukin-3 (IL3) family: IL3, IL5 and GMCSF (granulocyte-macrophage colony-stimulating factor receptor).
Interleukin-12 (IL12) family: IL12 and IL23.
Hormones and other haematopoietic growth factors family: GCSF (granulocyte colony-stimulating factor), EPO (erythropoietin), TPO (thrombopoietin, gene THPO), GH (growth hormone), prolactin (PRL) and leptin (LEP or OBS).
Type II contains interferons and other interleukin subfamilies: IL10 family (IL10, IL22, IL26) and IL20 family (IL19, IL20, IL24).
The cytokines each have many functions and their physiological actions are not limited to a unique tissue but are often ubiquitous and pleiotropic. These functions are also often redundant, so that several cytokines may exert the same effect of some target tissues or cells. The precise role of all cytokines will not be presented in detail, and the reader is referred to immunology and haematology handbooks. We will essentially focus this chapter on haematopoietic growth factors because of their role in cell proliferation and haematological malignancies and on immunity- and inflammation-related processes because of their potential role in oncogenesis.
4.2 Cytokine Receptors and JAK Activation
Cytokine receptors are membrane proteins with a single transmembrane domain and an extracellular N-terminal portion. They are characterised by the presence of cysteine residues in the distal part of the extracellular domain and by a motif containing two tryptophan and two serine residues (WSXWS) on the proximal part of this domain. The intracellular domain does not contain a catalytic centre but several well-conserved domains, in particular box 1, close to the transmembrane domain, characterised by a proline-rich motif of eight amino acids, and box 2, consisting of a group of hydrophobic amino acid residues in the prolongation of the α helix of the transmembrane domain, followed by a series of charged amino acid and tyrosine residues able to bind a phosphate moiety. The two boxes are involved in receptor binding to a JAK protein. One of these receptors, CNTFR, has no transmembrane and intracellular domains and is anchored to the plasma membrane through a molecule of glycosylphosphatidylinositol (Annex C). Several receptors present soluble variant isoforms generated by alternative splicing: this is the case for the receptors of IL4, IL5, IL7, IL9, LIF, GCSF, CNTF, GH and PRL. These soluble forms behave as cytokine transporters and some of them merely as traps, which bind a cytokine without transmitting a signal.
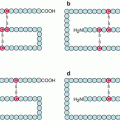
Cytokine receptors must be dimerised to activate the signalling pathway. The dimers and their ligand constitutes the ‘reception complex’ characteristic of the message to be transmitted. This can be a heterodimerisation between a ligand-specific monomer (α type) and a common, ligand family-specific, monomer (β or γ type). The most frequently used ligand family-specific receptors are IL3Rβ or βc for the GMCSF family, IL2Rγ or γc for the IL2 family and gp130 or IL6ST (IL6 signal transducer) for the IL6 family (Table 4.1). Heterodimerisation also occurs for the receptors of type II cytokines of the IL10 and IL20 families, with IL10Rβ and IL20Rβ as common receptors for these interleukins, respectively. In contrast, for the hormone (GH, PRL, etc.) receptors and the other haematopoietic cytokine (EPO, TPO) receptors, there exists only one type of receptors that is homodimerised upon ligand binding. A total of 36 reception complexes have been thus identified and Fig. 4.1 presents some of them.
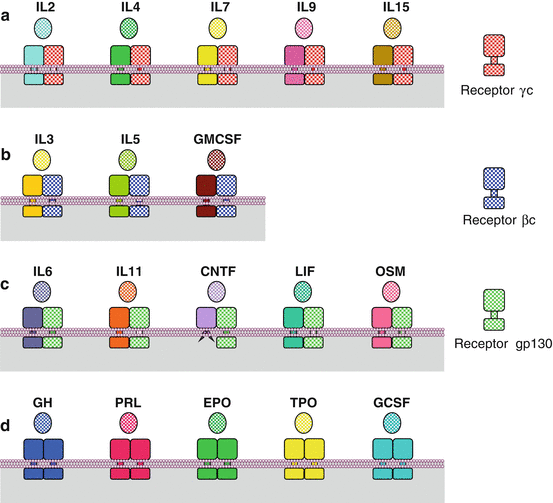

Fig. 4.1




Signal reception complexes of the main type I cytokines. The receptor dimers formed after recognition and binding of a cytokine are diverse. (a) Dimerisation occurs between an α receptor (specific to the cytokine) and a γc receptor (IL2Rγc, common to the IL2 cytokine family). (b) Dimerisation occurs between an α receptor (specific to the cytokine) and a βc receptor (IL3Rβc, common to the IL3 cytokine family). (c) Dimerisation occurs between an α receptor (specific to the cytokine) and a gp130 receptor (IL6ST) (common to the IL6 cytokine family). (d) Dimerisation occurs between two identical cytokine-specific receptors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree