Although most patients with Hodgkin lymphoma (HL) are cured with primary therapy, patients with primary refractory disease or relapse after initial treatment have poor outcomes and represent an unmet medical need. Recent advances in unraveling the biology of HL have yielded a plethora of novel targeted therapies. This review provides an overview of the data behind the hype generated by these advances and addresses the question of whether or not clinically these targeted therapies offer hope for patients with HL.
Key points
- •
Patients with Hodgkin lymphoma (HL) with primary refractory disease or relapse after transplant have poor outcomes and represent an unmet need.
- •
Therapies derived from an understanding of HL biology can be broadly classified as targeting the Hodgkin Reed Sternberg cell-surface receptors, tumor microenvironment, cell-mediated immunity, and intracellular signaling pathways.
- •
Brentuximab vedotin, an antibody-drug conjugate targeting CD30 now approved by the Food and Drug Administration, offers substantial hope for improving outcomes in the treatment of HL.
- •
Other therapies in development need longer follow-up to realize their potential.
Objectives
- 1.
Review recent advances in HL biology
- 2.
Review development of novel targeted therapies in the context of HL biology
- 3.
Review results of clinical trials with targeted therapies
Introduction
Classic Hodgkin lymphoma (HL) represents approximately 10% of all lymphomas diagnosed annually in the developing world. In 2013, approximately 9000 cases of HL were diagnosed in the United States. With a median age of 38 years, and at least 40% of patients younger than 35 years at the time of diagnosis, it is the most common lymphoma affecting young patients. Over the past 30 years, valuable lessons learned about the late effects of therapy, specifically cardiovascular and second cancer risk, have led to treatment modifications of radiation dose and field size as well as alkylator exposure, which have led to significant risk reduction of competing causes of death. As a result of these advances, more than 75% of patients are cured with contemporary frontline therapy.
For patients who relapse after attaining an initial complete remission (CR) or have primary refractory disease, the standard treatment approach is salvage chemotherapy followed by autologous stem cell transplant (ASCT), with an approximately 50% cure rate. Several studies show that achieving a CR before ASCT is one of the most important factors in determining a long-term outcome after ASCT. Other pretransplant prognostic factors include duration of initial remission, extent of disease at relapse, and constitutional symptoms. In an international collaborative effort from 5 countries, data on 756 patients with relapsed HL with a minimum of 1-year follow-up after the transplant were pooled. The overall median postprogression survival (PPS) for patients relapsing after ASCT was 1.3 years. Seventy-one percent of relapses occurred within 1 year after ASCT and were roughly equally distributed in the following periods: less than 3 months (22%), more than 3 and less than 6 months (22%), and more than 6 and less than 12 months (27%). The median PPS for these periods were 0.55, 1.6, 1.68, and 2.26 years for the time to relapse after ASCT, respectively ( P <.0001). Allogeneic stem cell transplantation (alloSCT) can induce durable remissions in some of these patients; however, its utility is limited by the challenges of finding an available stem cell donor, and achieving adequate disease control before transplantation. Therefore novel treatments to increase the CR rate pre SCT, or significantly prolong remission duration post SCT, have been sought.
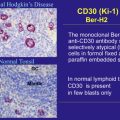
The recent approval in 2011 of brentuximab vedotin, an antibody-drug conjugate (ADC) targeting CD30, has been the first major advance in the management of HL after several decades and offers considerable hope to patients with refractory disease or relapse after stem cell transplant. Better understanding of the biology of HL has led to the exploration of several other potential targets as therapeutic options. This review provides an overview of HL tumor biology in the context of the development of novel targeted therapies. The authors discuss 4 broad categories of targeted therapies either approved or under investigation: (1) therapies targeting Hodgkin Reed Sternberg (HRS) cell-surface receptors, (2) therapies targeting reactive immune cells in the tumor microenvironment, (3) adoptive immunotherapy, and (4) therapies targeting signaling and intracellular survival pathways ( Tables 1 and 2 ). Although some of the agents discussed later are highly active as single agents, many others demonstrate modest single-agent activity. Moving forward, the challenge will be how to develop rational combinations of these novel agents within the context of current paradigms of care to achieve enhanced efficacy with minimal toxicity.
| Drug/Phase | Main Target | Clinical Trial Number | Failed ASCT (%) | Clinical Results | Reference |
|---|---|---|---|---|---|
| Receptor Targeted Therapies | |||||
| SGN-30 (I) | CD 30+ HRS cells | NCT00051597 | 83 a | No significant response | |
| SGN-30 (II) | NCT00337194 | 68 | |||
| MDX-060 (1/2) | CD 30+ HRS cells | NCT00284804 | 87 a | No significant response | |
| BV | |||||
| BV/(I) | CD 30+ HRS cells | NCT00947856 | 73 a | PIVOTAL Trial: ORR 75%, CR 34%, median | |
| BV/(I) | NCT01100502 | 68 a | PFS 5.6 mo, median DOR 20.5 mo | ||
| BV/(II) | NCT01060904 | 100 | — | ||
| HCD122 (II) | CD40+ HRS cells; Th2/Treg signaling | NCT00670592 | NR | ORR 16% (all PR) | |
| Galiximab (II) | CD80+ HRS cells | NCT00516217 | 83 | ORR of 6.9%, TTP 1.6 mo | |
| Microenvironment Targeting | |||||
| Lenalidomide (II) | Immunomodulation, antiangiogenesis | NCT00540007 | 76 | ORR 19% (N = 32) | |
| Lenalidomide (II) | NCT00478959 | 67 | ORR 13% (N = 15) | ||
| AFM 13 (I) | CD 16/30+ HRS cells | NCT01221571 | NR | 7% PR/50% SD | |
| Rituximab single agent (I pilot) | CD20+ peritumoral B lymphocytes CD20+ HRS cells | — | 82 | ORR 22%, median DOR 8.7 mo | |
| Rituximab + gemcitabine (II) | — | 55 | ORR 48%, median FFS 2.7 mo | ||
| Rituximab + ABVD frontline (I) | NCT00504504 | 0 | EFS 83% and OS 96% | ||
| Rituximab + ABVD frontline (II) | NCT00369681 | 0 | EFS 83% and OS 98% | ||
| PLX3397 (II) | CSF1R inhibitor | NCT01217229 | NR | ORR 5% | |
| Adoptive Immunotherapy | |||||
| EBV+ specific cytotoxic T cells | EBV+ HRS cells | NCT00058617 a | 40 | 83% of 28 patients with EBV+ HL had a clinical response, including 4 CRs sustained >9 mo | |
| Downstream Signaling Pathway | |||||
| Panobinostat (I) | Histone modification | NCT00742027 | 100 | ORR 27% including 4% CR, median PFS was 6.1 mo | |
| Vorinostat (I) | Histone modification, STAT signaling (pSTAT6) | NCT00132028 | 44 | ORR 4% | |
| Mocetinostat (I) | Histone modification, STAT signaling | NCT00358982 | 84 | ORR 21% | |
| Everolimus (I) | PI3K signaling, mTOR, TNFR signaling | NCT01022996 | 84 | ORR 47% 8 PR, 1 CR median TTP 7.2 mo, 4 responders remained progression free at 12 mo | |
| SB1518 | JAK/STAT pathway | NCT01263899 | NR | No significant clinical activity | |
| Drug | Main Target | Clinical Trial Number |
|---|---|---|
| Receptor Targeted Therapies | ||
| BV combinations | ||
| Frontline | ||
| Phase 3 frontline with AVD vs brentuximab/AVD | CD 30+ HRS cells | NCT01712490 |
| ECAPP B vs ECADD B (frontline) | NCT01569204 | |
| Relapsed/Refractory | ||
| ABVD→ BV (relapsed) | NCT01578967 | |
| BV + bendamustine (relapsed) | NCT01874054 | |
| BV + ipilimumab (relapsed) | NCT01896999 | |
| BV vs ICE pre ASCT (relapsed) | NCT01393717 | |
| BV→ ICE (relapsed) | NCT01508312 | |
| BV + rituximab (relapsed) | NCT01900496 | |
| Maintenance | ||
| BV maintenance after ASCT (ATHERA) (maintenance) | NCT01620229 | |
| TNX-650 | IL-13 | NCT00441818 |
| Microenvironment Targeting | ||
| Lenalidomide Combinations (relapsed) | ||
| AVD | Immunomodulation, antiangiogenesis | NCT01056679 |
| Bendamustine | NCT01412307 | |
| Romidepsin | NCT01742793 | |
| Everolimus | NCT01075321 | |
| Rituximab Combinations | ||
| Frontline | ||
| Rituximab ABVD vs ABVD phase 2 | CD20+ peritumoral B lymphocytes; CD20+ HRS cells | NCT00654732 |
| Rituximab + BEACOPP (HD18) | NCT00515554 | |
| Relapsed | ||
| Rituximab + Bendamustine | NCT01900496 | |
| Ipilimumab (relapsed) | Immunomodulation of tumor microenvironment | NCT01896999 |
| Nivolumab a (relapsed) | PD-1 expressing peritumoral lymphocytes | NCT01592370 |
| CDX1127 (relapsed) | Anti-CD27 antibody | NCT01460134 |
| Adoptive Immunotherapy | ||
| Autologous CAR.CD30 EBV specific-cytotoxic T-lymphocytes (relapsed) | EBV+ CD30+ HRS cells; CD30+ HRS cells | NCT01192464 |
| Downstream Signaling Pathways | ||
| MLN4924 (relapsed) | NFκB via inhibition of Ned8 | NCT00722488 |
| Everolimus (relapsed) | ||
| Everolimus and panobinostat | PI3K signaling, mTOR, TNFR signaling | NCT00918333 |
| Everolimus and lenalidomide | NCT01075321 | |
Introduction
Classic Hodgkin lymphoma (HL) represents approximately 10% of all lymphomas diagnosed annually in the developing world. In 2013, approximately 9000 cases of HL were diagnosed in the United States. With a median age of 38 years, and at least 40% of patients younger than 35 years at the time of diagnosis, it is the most common lymphoma affecting young patients. Over the past 30 years, valuable lessons learned about the late effects of therapy, specifically cardiovascular and second cancer risk, have led to treatment modifications of radiation dose and field size as well as alkylator exposure, which have led to significant risk reduction of competing causes of death. As a result of these advances, more than 75% of patients are cured with contemporary frontline therapy.
For patients who relapse after attaining an initial complete remission (CR) or have primary refractory disease, the standard treatment approach is salvage chemotherapy followed by autologous stem cell transplant (ASCT), with an approximately 50% cure rate. Several studies show that achieving a CR before ASCT is one of the most important factors in determining a long-term outcome after ASCT. Other pretransplant prognostic factors include duration of initial remission, extent of disease at relapse, and constitutional symptoms. In an international collaborative effort from 5 countries, data on 756 patients with relapsed HL with a minimum of 1-year follow-up after the transplant were pooled. The overall median postprogression survival (PPS) for patients relapsing after ASCT was 1.3 years. Seventy-one percent of relapses occurred within 1 year after ASCT and were roughly equally distributed in the following periods: less than 3 months (22%), more than 3 and less than 6 months (22%), and more than 6 and less than 12 months (27%). The median PPS for these periods were 0.55, 1.6, 1.68, and 2.26 years for the time to relapse after ASCT, respectively ( P <.0001). Allogeneic stem cell transplantation (alloSCT) can induce durable remissions in some of these patients; however, its utility is limited by the challenges of finding an available stem cell donor, and achieving adequate disease control before transplantation. Therefore novel treatments to increase the CR rate pre SCT, or significantly prolong remission duration post SCT, have been sought.
The recent approval in 2011 of brentuximab vedotin, an antibody-drug conjugate (ADC) targeting CD30, has been the first major advance in the management of HL after several decades and offers considerable hope to patients with refractory disease or relapse after stem cell transplant. Better understanding of the biology of HL has led to the exploration of several other potential targets as therapeutic options. This review provides an overview of HL tumor biology in the context of the development of novel targeted therapies. The authors discuss 4 broad categories of targeted therapies either approved or under investigation: (1) therapies targeting Hodgkin Reed Sternberg (HRS) cell-surface receptors, (2) therapies targeting reactive immune cells in the tumor microenvironment, (3) adoptive immunotherapy, and (4) therapies targeting signaling and intracellular survival pathways ( Tables 1 and 2 ). Although some of the agents discussed later are highly active as single agents, many others demonstrate modest single-agent activity. Moving forward, the challenge will be how to develop rational combinations of these novel agents within the context of current paradigms of care to achieve enhanced efficacy with minimal toxicity.
| Drug/Phase | Main Target | Clinical Trial Number | Failed ASCT (%) | Clinical Results | Reference |
|---|---|---|---|---|---|
| Receptor Targeted Therapies | |||||
| SGN-30 (I) | CD 30+ HRS cells | NCT00051597 | 83 a | No significant response | |
| SGN-30 (II) | NCT00337194 | 68 | |||
| MDX-060 (1/2) | CD 30+ HRS cells | NCT00284804 | 87 a | No significant response | |
| BV | |||||
| BV/(I) | CD 30+ HRS cells | NCT00947856 | 73 a | PIVOTAL Trial: ORR 75%, CR 34%, median | |
| BV/(I) | NCT01100502 | 68 a | PFS 5.6 mo, median DOR 20.5 mo | ||
| BV/(II) | NCT01060904 | 100 | — | ||
| HCD122 (II) | CD40+ HRS cells; Th2/Treg signaling | NCT00670592 | NR | ORR 16% (all PR) | |
| Galiximab (II) | CD80+ HRS cells | NCT00516217 | 83 | ORR of 6.9%, TTP 1.6 mo | |
| Microenvironment Targeting | |||||
| Lenalidomide (II) | Immunomodulation, antiangiogenesis | NCT00540007 | 76 | ORR 19% (N = 32) | |
| Lenalidomide (II) | NCT00478959 | 67 | ORR 13% (N = 15) | ||
| AFM 13 (I) | CD 16/30+ HRS cells | NCT01221571 | NR | 7% PR/50% SD | |
| Rituximab single agent (I pilot) | CD20+ peritumoral B lymphocytes CD20+ HRS cells | — | 82 | ORR 22%, median DOR 8.7 mo | |
| Rituximab + gemcitabine (II) | — | 55 | ORR 48%, median FFS 2.7 mo | ||
| Rituximab + ABVD frontline (I) | NCT00504504 | 0 | EFS 83% and OS 96% | ||
| Rituximab + ABVD frontline (II) | NCT00369681 | 0 | EFS 83% and OS 98% | ||
| PLX3397 (II) | CSF1R inhibitor | NCT01217229 | NR | ORR 5% | |
| Adoptive Immunotherapy | |||||
| EBV+ specific cytotoxic T cells | EBV+ HRS cells | NCT00058617 a | 40 | 83% of 28 patients with EBV+ HL had a clinical response, including 4 CRs sustained >9 mo | |
| Downstream Signaling Pathway | |||||
| Panobinostat (I) | Histone modification | NCT00742027 | 100 | ORR 27% including 4% CR, median PFS was 6.1 mo | |
| Vorinostat (I) | Histone modification, STAT signaling (pSTAT6) | NCT00132028 | 44 | ORR 4% | |
| Mocetinostat (I) | Histone modification, STAT signaling | NCT00358982 | 84 | ORR 21% | |
| Everolimus (I) | PI3K signaling, mTOR, TNFR signaling | NCT01022996 | 84 | ORR 47% 8 PR, 1 CR median TTP 7.2 mo, 4 responders remained progression free at 12 mo | |
| SB1518 | JAK/STAT pathway | NCT01263899 | NR | No significant clinical activity | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree