Fig. 4.1
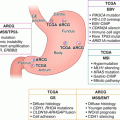
The BRCA1 pathway in response to DNA double-stand breaks. SU: SUMOylation; P: Phosphorylation (Adapted from Differential effect of MMSET mRNA levels on survival to first-line FOLFOX and second-line docetaxel in gastric cancer (2014), Wei J et al. [42])
In previous studies, we have analyzed mRNA expression levels of BRCA1, RAP80, PIAS1, PIAS4, MDC1, UBC9, 53BP1, and MMSET in advanced gastric cancer patients [41, 42]. All patients received first-line FOLFOX chemotherapy treatment and exhibited disease progression, with 59 patients treated with second-line docetaxel-based chemotherapy. Among those patients that received only first-line oxaliplatin-based chemotherapy, the median survival was determined to be 12.3 months for those with low levels of MMSET, compared to 8.8 months for those with high levels of MMSET (p = 0.04). The median survival time was found to be 12.4 months for patients with low levels of UBC9 and 8.8 months for patients with high levels of UBC9 (p = 0.01). Longer survival was also observed in patients with low levels of BRCA1 (p = 0.20), MDC1 (p = 0.49), and 53BP1 (p = 0.09). However, the differences were not found to be significant. Among patients with low MMSET expression levels, the median survival time was 19.9 months for patients with low 53BP1 levels and 5.9 months for those with high 53BP1 levels (p = 0.02). Multivariable analyses were carried out in patients that received only first-line treatment. A decreased mortality risk was observed in patients with low MMSET levels (HR, 0.59; 95% CI, 0.35–0.98; p = 0.04) and in patients with low UBC9 levels (HR, 0.52; 95% CI, 0.31–0.88; p = 0.01). Differential modulators of survival were observed in several genes and gene combinations, an observation which will be discussed in the section regarding taxanes.
In addition to DNA repair genes, certain miRNAs impact the sensitivity of a patient toward chemotherapy treatment if their levels are artificially upregulated. This is similar to the data regarding miRNAs with diagnostic potential. An upregulation of miR-21 or miR-106a was demonstrated to result in an increase in cisplatin resistance of GC cells [43, 44]. However, the upregulation of miR-449 was demonstrated to have a positive impact on sensitivity toward cisplatin [45].
4.3.2 5-Fluorouracil (5-FU)/Capecitabine/S-1
5-FU and its oral forms are the primary chemotherapeutic drugs used in the treatment of gastric cancer patients. Capecitabine is hepatically metabolized and thus ultimately converted into 5-FU at the tissue level [46]. Another oral fluoropyrimidine, notably S-1, possesses tegafur as the active moiety. This is transformed to 5-FU by cytochrome p450 in the liver [47]. 5-FU is an S phase-specific agent which exhibits cytotoxicity through the incorporation of fluoronucleotides into RNA and DNA molecules. 5-FU is converted into its active metabolite, fluorodeoxyuridine monophosphate (FdUMP), by thymidine phosphorylase (TP) and orotate phosphoribosyltransferase (OPRT). FdUMP exhibits a high affinity for thymidylate synthase (TS), the primary target of fluoropyrimidines. Methylenetetrahydrofolate reductase (MTHFR) plays a critical role in both fluoropyrimidine synthesis and the regulation of folate intracellular flow. In addition, 5-FU is phosphorylated by orotate phosphoribosyl transferase, resulting in the inhibition of RNA synthesis. The rate-limiting enzyme in 5-FU catabolism is dihydropyrimidine dehydrogenase (DYPD). DYPD is also involved in the conversion of the oral pro-drug capecitabine to 5-FU at the cellular level. TP activity in cancer cells has been correlated with the intra-tumoral 5-FU concentration following capecitabine administration [48]. Research studies related to 5-FU pharmacogenetics and pharmacogenomics primarily focused on several genes, including TS, TP, MTHFR, OPRT, and DYPD.
TS is the most extensively studied enzyme. A seminal study carried out in 1996 demonstrated that TS mRNA levels influence the response to 5-FU-based chemotherapy, and thus survival, in a cohort of patients with primary gastric cancer [49]. Numerous following studies validated this negative predictive role for TS expression in response to fluoropyrimidines [48]. However, in nonmetastatic cases, some studies have not identified any correlation between low TS expression and a response to 5-FU. This has been explained by a possible prognostic role of TS, which could be involved in tumor progression rather than the chemotherapy response in the case of these negative studies [50–52].
A meta-analysis demonstrated that polymorphisms in TS and MTHFR were closely associated with the clinical outcomes of GC patients treated with 5-FU-based chemotherapy [53]. However, the effect of TS polymorphisms could vary through ethnicity stratification due to different allelic distributions [54].
The biomarker study carried out in the SPIRITS trial demonstrated the effect of fluorouracil-metabolizing enzymes on the outcomes of patients treated with S-1 alone or S-1 plus cisplatin (CS) as the first-line treatment in advanced gastric cancer. The mRNA levels of TS, TP, OPRT, DYPD, vascular endothelial growth factor-A (VEGFRA), and epidermal growth factor receptor (EGFR) were studied in paraffin-embedded specimens isolated from primary tumors. Multivariate survival analysis in patients that received S-1 monotherapy demonstrated that low TP expression, low TS, and high OPRT were significant predictors of long overall survival. In patients with lower expression levels of both TP and TS, the S-1 alone group demonstrated longer overall survival compared to the CS group. However, the frequency of overall adverse events in the S-1 treatment alone group tended to be lower than that in CS treatment group [55].
Another study which examined biomarkers related to capecitabine, platinum, and taxane therapy has demonstrated the role of four key target or metabolic enzymes: class III β-tubulin (TUBB3), TS, TP, and ERCC1. As described earlier, both TS and TP are key metabolic enzymes of capecitabine. TUBB3 and ERCC1 overexpression was shown to indicate a resistance to taxanes and platinum, respectively. The response rates of patients to capecitabine plus paclitaxel or capecitabine plus cisplatin treatment were observed to be markedly different between patients exhibiting low TUBB3/high TP expression levels (87.5%) compared with those exhibiting high TUBB3/low TP expression levels (14.3%) (p = 0.01). Similarly, the response rate was determined to be 57.9% for the low TS/high TP subgroup and 15.8% for the high TS/low TP subgroup (p = 0.007) [56].
In addition to 5-FU metabolic enzymes, the regenerating gene family (REG) was reported to play a role in chemosensitivity. In vitro studies have demonstrated that induction of REG Iα gene expression confers resistance to 5-FU or CDDP treatment in GC cells. In patients with stage IV GC, REG Iα could be a potential biomarker for predicting resistance to S-1/CDDP treatment [57]. In addition, high REG IV serum levels in gastric cancer patients was identified as predictive measure for the resistance to 5-fluorouracil-based chemotherapy [58].
4.3.3 Taxanes
Taxanes, as its name indicates, were first derived from the plants, Taxus. Taxane agents bind and stabilize microtubule, leading to tumor cell cycle arrest at the G2-M phase [59]. Taxanes have achieved definitive curative effects either alone or in combination with other chemotherapy drugs as both first- and second-line therapy in the treatment of gastric cancer. There are four primary types of taxane agents, including paclitaxel, docetaxel, nanoparticle albumin-bound paclitaxel (nab-paclitaxel), and cabazitaxel. Paclitaxel and docetaxel belong to the taxane family, as their chemical structures contain a common three-phenol ring. Nab-paclitaxel is a novel drug that is a biologically interactive form of paclitaxel. Cabazitaxel is second-generation taxane which is currently being used in ongoing phase II trials (NCT01757171, NCT01956149) to illustrate its role in the treatment of gastric cancer.
According to published reports, sensitivity to taxanes involves multiple mechanisms, including drug efflux, mutations in tubulin, altered microtubule dynamics, and impaired cell death signaling [60].
Overexpression of the multidrug resistance (MDR) gene like MDR1, which encodes P-glycoprotein, resulted in taxane efflux, decreasing drug retention and thus causing taxane resistance [61].
Because taxane targets microtubules, attention has been directed toward the study of tubulin and microtubule-associated proteins. Mutations [62] and polymorphisms [63, 64] in β-tubulin have been shown to be associated with taxane resistance. The overexpression of β-tubulin isotypes was identified as another mechanism of resistance [65]. Numerous studies have validated the fact that taxane treatment is inversely correlated with mRNA and protein expression levels of βIII-tubulin in gastric cancer [66–68]. Furthermore, a recent report suggested that the interaction of vascular endothelial growth factor (VEGF) with βIII-tubulin through hypoxia-inducible factor (HIF-alpha) was associated with taxane sensitivity. VEGF inhibition, which blocks both VEGFR-1 and VEGFR-2, has been demonstrated to reverse paclitaxel sensitivity in gastric cancer cells [69]. In addition, the sensitivity of gastric cancer patients to paclitaxel treatment was found to be inversely correlated with mRNA and protein expression levels of the microtubule-associated protein, tau (MAPT) [66, 70]. Chemokine receptor-4 (CXCR4) was identified to play a role in microtubule dynamics. CXCR4 mRNA levels in gastric cancer tissues were also found to correlate with docetaxel sensitivity [71].
The induction of apoptosis by taxanes has been shown to be mediated through the mitochondrial apoptotic pathway, typically by members of the Bcl2 family [72]. The pro-apoptotic protein, BIM, has been demonstrated to translocate from microtubules to mitochondria following taxane treatment [73]. Thus, cancer cell lines with higher BIM expression levels were found to be more sensitive to taxanes compared to cells that expressed lower levels of BIM [60]. In addition to antiapoptotic properties, glucose-regulated protein 78(GRP78) overexpression was found to be a predictive marker for the development of taxane-based therapeutic resistance [74]. Survivin is a member of the family of inhibitor of apoptosis proteins (IAPs), and its expression levels have been demonstrated to be inversely correlated with taxane treatment [66].
Numerous studies have shown that Forkhead box protein M1 (FOXM1), which plays an important role in cell cycle regulation, mediates docetaxel resistance in gastric cancer patients [75, 76]. In addition, docetaxel resistance was shown to be reversed upon inhibition of FOXM1 [77]. Furthermore, genes that play a role in the DNA damage response pathway, including BRCA1, have been shown to also play a critical role in a patient’s response to taxane agents. Tumors exhibiting high BRCA1 expression were found to exhibit an increased susceptibility to docetaxel [23]. As discussed earlier in the section regarding platinum, our study found that of 59 patients who received first-line FOLFOX and second-line docetaxel-based chemotherapy, the median overall survival was 25.8 months for patients with high BRCA1 expression levels, 19.1 months for patients with intermediate BRCA1 expression levels, and 9.5 months for those with low BRCA1 expression levels (p = 0.0062) [78].
In addition, previous studies have demonstrated that ErbB3 overexpression and AKT/ERK activation can induce gastric cancer cell resistance to paclitaxel treatment [79]. Homomeric α7-nicotinic acetylcholine receptor (A7-nAChR) was found to be a key modulator of smoking-induced gastric cancer metastasis. Interestingly, A7-nAChR knockdown cells were shown to exhibit higher sensitivity to paclitaxel treatment in gastric cancer cells [80].
4.3.4 Irinotecan(CPT-11)
Irinotecan (CPT-11)-based treatment regimens were commonly used for the treatment of gastric cancer. This treatment exhibited a good response rate in patients, varying from 14–70% when used as a single or combination treatment agent [81].
Irinotecan is a semisynthetic, water-soluble derivative of the plant alkaloid camptothecin. This compound belongs to the class of topoisomerase I inhibitors. Addition of irinotecan to the topoisomerase I (Topo I)-DNA complex obstructs the ligation of double-stranded DNA during the process of DNA replication. This causes Topo I to be trapped on a nicked DNA intermediate in replicating cells, resulting in cell death [82]. A variety of DNA repair genes, including aprataxin (APTX), BRCA1, ERCC1, and ATM, are involved in the repair of irinotecan-associated DNA damage. Studies have reported that high gene expression levels of Topo I are associated with irinotecan sensitivity in gastric cancer [83]. Interestingly, significantly lower gene expression levels of APTX, BRCA1, and ERCC1 have been reported to be associated with irinotecan-sensitive gastric cancer samples, compared with that of irinotecan-resistant samples [84]. Furthermore, low expression levels of the DNA repair gene, ATM kinase, were also found to be associated with increased irinotecan drug sensitivity in gastric cancer cell lines [85]. Consistent results were observed in the molecular biomarker study of GC0301/TOP-002 phase III trials in gastric cancer. The results of these trials show that low TS, low ERCC1, and high TP mRNA levels function as biomarkers for irinotecan treatment [83].
Recently, gene hyper-methylation has been demonstrated to be an important epigenetic mechanism of drug response in gastric cancer [86]. Methylation of the oncogene, heparan sulfate 6-O-endosulfatase (SULF2), and methylation of the tumor-suppressor gene, WRN, were both reported to render gastric cancer sensitive to irinotecan treatment [87].
4.3.5 Pemetrexed
Pemetrexed is a new antifolate drug that has been shown to target multiple components within the folate pathway, including thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT) [90]. Pemetrexed was demonstrated to mediate its antitumor effect by impeding both DNA synthesis and folate metabolism [91]. Clinical antitumor activity of pemetrexed in gastric cancer has also been observed in several clinical trials where it has been used either as a single agent or in combination with another chemotherapy agent. The response rate in these trials was found to range from 23–36% [92–95].
Thymidylate synthase (TS) is a critical enzyme that plays a role in the synthesis of DNA. Research from our group indicates that low expression levels of TS in the plasma and tumor were negatively correlated with pemetrexed sensitivity in gastric cancer patients (p < 0.001) [96]. TS levels in plasma and tumor were found to be lower in the pemetrexed-sensitive group compared to the pemetrexed-resistant group. Previous study also confirmed that low DHFR and GARFT gene expression levels exhibited a significant correlation with chemosensitivity to pemetrexed in freshly explanted tumor cells in vitro [97].
4.3.6 Other Regimens
Other regimens, such as doxorubicin, epirubicin, and mitomycin, could also function as effective treatments in gastric cancer patients.
Doxorubicin is a member of the anthracycline group of compounds and is known to exhibit activity against a wide range of tumors. The primary mechanism of action of this compound appears to be through inhibition of topoisomerase II. In addition, it has also been shown to be capable of forming adducts with DNA in order to induce cell death [98]. Studies have shown that high expression levels of the apoptosis repressor with caspase recruitment domain (ARC) in gastric cancer cell lines contribute to doxorubicin chemotherapy resistance [99]. Recent microarray data has enabled researchers to identify novel genes including ADAM22, CYR61, FN1, SPHK1, and GNAI1 as predictive markers of doxorubicin sensitivity in gastric cancer [100].
Epirubicin is another example of an antitumor agent of anthracyclines. This specific compound is thought to enter tumor cells more efficiently in order to exert its antitumor effects though the inhibition of nucleic acid synthesis and mitosis, both leading to cell death [101]. Studies have reported that high expression levels of MDR genes, including MDR1, MRP1, and ABCG2, in gastric cancer patients correlated with the development of resistance to epirubicin [102]. Gastric cancer patients with high expression levels of DNA synthesis-related genes, such as topoisomerase IIA (TOP2A), have been shown to benefit from epirubicin therapy [20]. Furthermore, amplification of human epidermal growth factor receptor-2 (HER-2) or HER-2 overexpression was identified as a potential biomarker to flag those patients who benefited from either perioperative or postoperative epirubicin-based therapy in gastroesophageal adenocarcinoma [103].
Mitomycin is a cell cycle nonspecific agent, an anticancer drug that is commonly used to treat numerous cancer types, including stomach, anal, and lung cancer [104]. Studies investigating the antitumor mechanism of mitomycin revealed that this drug induced DNA damage via DNA alkylation. This was found to result in the production of DNA mono-adducts, intrastrand cross-links, and interstrand cross-links (ICLs) [105, 106]. However, the specific mechanism underlying mitomycin resistance has not been fully understood. In vitro studies have revealed that TCF transcription factor 3 played a role in mitomycin resistance in gastric cancer patients [107]. AKT activation and loss of heterozygosity (LOH) of phosphatase and tensin homolog on chromosome ten (PTEN) were also found to be associated with mitomycin-related chemoresistance in GC patients [108]. Both normal and cancer cells that were found to lack the suppressor gene, FHIT, exhibited mitomycin C resistance [109].
4.3.7 Markers for Toxicity
Common adverse drug events of chemotherapy treatment for gastric cancer include nausea and vomiting, fatigue, diarrhea, liver and kidney dysfunction, blennophlogisma, and hematopoietic disorders such as granulocytopenia, thrombocytopenia, and anemia. Numerous studies have been carried out in an effort to discover the biomarkers that can be used to predict drug toxicity in chemotherapy treatments for gastric cancer [110].
Genetic polymorphisms were found to play a critical role in the pharmacologic activity of commonly utilized medications, which was found to contribute to the different responses observed to chemical agents. Polymorphisms in key components of the nucleotide excision repair (NER) pathway or drug metabolic pathways were found to be significantly associated with a higher drug toxicity incidence with platinum or S-1 treatment [111–114]. Polymorphic abnormalities in the human TS gene was also identified as a risk factor for serious adverse reactions to 5-FU-based therapy [115]. In addition, studies focused on polymorphisms in gene UGT1A1 revealed that patients who carry the UGT1A1*6 A/A allele or UGT1A1*28 variants experienced increased diarrhea and were more prone to developing hematopoietic disorders upon treatment with irinotecan [116–119]. DHFR F/S-TS G52S, which is a fusion gene of both mutant enzymes, dihydrofolate reductase (DHFR F/S) and thymidylate synthase (TS G52S), has recently been shown to possess the ability to confer resistance to pemetrexed-induced toxicity. Retroviral transduction to express this fusion gene in cells was found to result in a significantly higher pemetrexed IC50 and increased survival of CFU-GM colonies compared with those transduced with either of the mutants alone [120].
In addition to the genetic indicators, biochemical indicators in serum or urine could also function as valuable markers for predicting side effects from anticancer drugs. Serum diamine oxidase (DAO) activity, which reflects the integrity and maturity of the small mucosa, has been shown to function as an indicator of gastrointestinal damage prior to symptom onset in patients undergoing chemotherapy treatment [121]. Large clinical studies have shown that elevated baseline concentrations of both homocysteine and methylmalonic acid indicate severe hematological toxicity, as a result of pemetrexed treatment [122]. The presence of neutrophil gelatinase-associated lipocalin (NGAL) in urine is also widely accepted as an assessment of renal injury in patients receiving cisplatin treatment [123].
Conclusions
Biomarkers can be investigated at various levels. These include genetic analyses that identify polymorphisms, DNA sequencing, transcriptional assays such as reverse transcriptional-polymerase chain reaction (RT-PCR) that measure mRNA levels, and transductional tests, such as immunohistochemistry, that measure protein expression levels. However, having such a variety of available tools to identify biomarkers is not always advantageous. While this number of tools is advantageous in that it allows for a wider comprehension of biomarkers, it can also be misleading due to discrepancies obtained between different techniques. With the exception of HER-2 status for trastuzumab-based targeted treatment, no other molecular markers have entered the mainstream of clinical practice. The primary obstacle toward the identification of reliable markers lies in technical difficulties that arise in the ability to quantitatively assess molecular alterations. In addition, the use of a single biomarker allows only limited power toward predicting the prognosis or response to specific chemotherapy treatments. Thus, the most promising approach would entail the evaluation of a combination of variables in order to achieve a more reliable predictive value than using a single biomarker.
References
1.
Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–18. doi:10.1016/S1470-2045(15)00553-7.PubMed
2.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064. doi:10.1002/14651858.CD004064.pub3.
3.
Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004;22(4):658–63.PubMed
4.
Lordick F, Lorenzen S, Stollfuss J, Vehling-Kaiser U, Kullmann F, Hentrich M, et al. Phase II study of weekly oxaliplatin plus infusional fluorouracil and folinic acid (FUFOX regimen) as first-line treatment in metastatic gastric cancer. Br J Cancer. 2005;93(2):190–4.PubMedPubMedCentral
5.
Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer. 2004;91(3):453–8.PubMedPubMedCentral
6.
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92(9):1644–9.PubMedPubMedCentral
7.
Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(9):1435–42. doi:10.1200/JCO.2007.13.9378.
8.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531.PubMed
9.
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666–73. doi:10.1093/annonc/mdn717.PubMed
10.
Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20(9):1529–34. doi:10.1093/annonc/mdp047.PubMed
11.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(31):4991–7. doi:10.1200/JCO.2006.06.8429.
12.
Van Cutsem E, Boni C, Tabernero J, Massuti B, Richards DA, Prenen H, et al. Randomized phase II study (GATE study) of docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer. J Clin Oncol. 2011;29(S15):abstract 4018!!
13.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–21. doi:10.1016/S1470-2045(08)70035-4.PubMed
14.
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(9):1547–53. doi:10.1200/JCO.2009.25.4706.
15.
Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19(8):1450–7. doi:10.1093/annonc/mdn166.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree