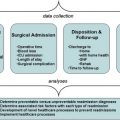
For most cancers, surgical therapy offers the only hope for cure. Nevertheless, evidence suggests wide variation in cancer care, and therefore it is imperative to ensure that high standards of care are being met. Few initiatives currently exist that are focused on cancer care quality, and there is no program measuring short-term surgical outcomes following cancer surgery. Improvements in care will likely come from performance programs that provide reliable, robust, and actionable information in a timely manner such that performance feedback can occur more frequently and at earlier stages in the treatment and disease process.
- •
For the majority of cancers, surgical therapy offers the only hope for cure. With the aging population and aggressive screening as well as neoadjuvant therapies, increasing numbers of patients will be eligible for surgical resection.
- •
Evidence suggests wide variation in cancer care quality in the United States.
- •
Few programs exist that focus on hospital cancer quality, and no program exists that targets hospital surgical quality with respect to short-term outcomes for cancer.
- •
Improvements in care will likely come from performance programs that provide reliable, robust, and actionable information in a timely manner, such that performance feedback can occur more frequently and at earlier stages in the treatment and disease process.
In 1999, the Institute of Medicine (IOM) released its sentinel publication “To Err is Human,” which led to an increasing awareness of deficiencies in patient safety in the United States, particularly in surgery. At the same time, the IOM published “Ensuring Quality Cancer Care,” which documented substantial variation in the quality of cancer care in the United States. These reports explicitly substantiated the need to consistently and reliably measure the quality of cancer care.
Nevertheless, despite significant effort and cost to develop and improve cancer care, evidence from the ensuing decade suggested that adherence to basic standards of care continued to remain unsatisfactory. For example, in 2003, McGlynn and colleagues documented that only 56% of surgical patients received basic standards of surgical processes of care in the United States. Three years later in 2006, Malin and colleagues evaluated adherence to standards of care in breast and colorectal cancer in 5 metropolitan areas across the United States. They reported an aggregated improvement; however, the variability remained high in certain domains. Specifically, the adherence in breast cancer care was 86% and colorectal cancer 78%, but, in breast cancer, for example, adherence to the 4 surgical quality-of-care measures varied widely from only 30% to nearly 100%.
Although it remains challenging, one approach to reducing this variability in the quality of cancer care is the development, implementation, and feedback of cancer-specific quality measures, such that hospitals can identify areas requiring needed improvements. As such, both governmental and private stakeholders of health care quality have increasingly become intimately involved with cancer care quality improvement specifically around the development of quality improvement initiatives. For example, the National Quality Forum (NQF) launched a program to encourage the development of cancer-specific quality measures, such as ensuring adequate lymph nodes are removed and examined and appropriate postoperative therapies are provided after colectomy for cancer. In addition, recent legislation in the Affordable Care Act provides a clear mandate for performance evaluation, including the requirement of cancer care performance assessment. Also, in late 2010, the Centers for Medicare and Medicaid Services (CMS) announced a contract with 2 private organizations to develop up to 6 cancer-specific quality-of-care measures for eventual use in public reporting. Thus, given the growing attention and importance of quality measurement in surgical oncology, the objectives of this article are (1) to discuss currently available quality measures and initiatives and (2) to explore the specific programs currently available for measuring the quality of cancer care.
Defining quality cancer care
The IOM defines quality health care as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.” However, defining quality cancer care has proved challenging. Caring for a single patient with cancer requires the coordinated effort of a diverse set of providers and health systems. Traditional definitions of quality measurement use the Donabedian framework of structure, process, and outcomes. Structure refers to the system in which the care is delivered such as intensive care unit nurse to patient ratios or presence of interventional radiologists. Processes of care are related to characteristics of the actual care delivered, for example, performing guideline-recommended lymph node examinations or treating patients with stage III colon cancer with adjuvant chemotherapy. Outcomes are often considered the bottom line of quality care; however, there is some debate regarding whether short-term or long-term outcomes should be emphasized. Moreover, measuring outcomes requires rigorous risk adjustment using high-quality data and is heavily dependent on the specific methodology used.
Nevertheless, the Donabedian model of quality may not completely encompass the full spectrum of cancer-specific health care quality. Other metrics may be equally or more important. Examples include the accessibility and timeliness of care, cancer-related health care costs, quality-of-life metrics, and the patient centeredness of cancer care. Despite the growing body of knowledge reflecting what constitutes high-quality care, a fundamental challenge lies in determining how to identify poor performance and translate this information into meaningful, real-world changes in clinical practice. The first step may be developing standardized and reliable quality measures and benchmarks of care.
Currently available cancer quality measures
Despite the definitional complexity of what constitutes quality cancer care, hospitals and providers must ultimately be evaluated and judged as optimal or in need of improvement. To this end, both private and public organizations, including the NQF, Agency for Healthcare Research and Quality, CMS, the Joint Commission, and the American Hospital Association, have been instrumental in motivating the development of more than 100 measures and benchmarks of care specific to cancer. The NQF has perhaps taken the lead by rigorously and thoroughly evaluating measures based on a set of predefined standards, including clinical importance, scientific acceptability, usability, and feasibility of data collection.
NQF
In 2002, the NQF released an initial call for measures to identify opportunities for health care professionals to improve cancer care. This initiative focused on breast, colorectal, and end-of-life cancer care and resulted in 19 NQF-endorsed performance measures. Breast cancer measures related to surgery included post–breast-conserving surgery irradiation, use of adjuvant chemotherapy or hormone therapy, adherence to the College of American Pathologists Breast Cancer Protocol, use of needle biopsy for diagnosis, and evaluation of axillary lymph nodes in early-stage disease. Colorectal cancer measures included adjuvant chemotherapy use, completeness of pathologic reporting, adherence to the College of American Pathologists Colon and Rectum Protocol, and the examination of at least 12 lymph nodes. Because CMS requires NQF endorsement before their use in public reporting or payment incentives, many of these measures are or will soon be implemented at the national scale.
American Society of Clinical Oncology
In a separate program led by the American Society of Clinical Oncology (ASCO) in conjunction with RAND Corporation and Harvard University, health service researchers conducted the National Initiative on Cancer Care Quality study. Findings from this report identified no surgery-specific measures. Nevertheless, treatment-related measures included optimizing chemotherapy dosing, managing side effects, advising patients about treatment options, better documentation of key cancer staging and treatment details, and ensuring that patients at the highest risk of poor outcomes receive the recommended care.
Other Available Measures
Although most measures reflect breast and colorectal cancers, health services researchers have also identified several quality measures in other cancer sites. In a 2009 study focusing on melanoma care, 26 measures were identified as valid according to RAND/UCLA Appropriateness Methodology. Twenty-four of these measures were processes of care and were mainly related to receiving guideline treatments such as ensuring negative margins, examining appropriate numbers of lymph nodes, and undergoing standard preoperative workup algorithms. A separate report focused on developing pancreatic cancer quality indicators that also used RAND/UCLA Appropriateness Methodology. Forty-three indicators were identified as valid, of which 11 were structural, 19 were process related, 4 addressed treatment appropriateness, 4 assessed efficiency, and 5 assessed outcomes.
Gaps in Cancer Quality Measures
Although many measures have been developed, several gaps exist that need to be addressed. First, of the measures currently available, many are not disease specific or focus only on the most common cancers, such as the NQF-endorsed colorectal and breast cancer quality measures. It should be emphasized that although other cancers are less prevalent, they often have even greater variations in quality. For example, with respect to lymph node examinations following cancer surgery, adherence to the 12–lymph node measure is much better in colon cancer than other common cancers such as esophagus, gastric, and pancreas cancers.
Second, there is a lack of surgical outcome assessment in endorsed cancer care quality measures. This is not surprising, given that outcome measures can be considerably more difficult to develop and implement and are at times seen as threatening evaluations. Unlike processes of care, outcomes require the collection of standardized and reliable data for appropriate risk adjustment to account for differences in patient, disease, and procedural risk. Nevertheless, risk-adjusted outcome comparisons may be better reflections of true hospital quality. Recently, the NQF endorsed the first colon resection outcome quality measure, which was developed by the American College of Surgeons (ACS). This measure was demonstrated to be feasible, reliable, and accurate, and, although not intended to be used only after resection for cancer, recent work suggests that cancer-specific variables may not be necessary for robust risk-adjusted hospital quality short-term outcome assessment.
Last, other quality domains that have not been sufficiently addressed include timeliness of care (eg, time from diagnosis to surgery, time for surgical resection to adjuvant chemotherapy in stage III colon cancer) and appropriateness of care (eg, appropriateness of axillary dissection in selected patients with low-risk breast cancer). Nevertheless, these domains are not yet clearly defined, and further research is required before their endorsement and implementation.
Despite the numerous quality indicators currently in existence, without actionable data they cannot affect a meaningful improvement for individual patients with cancer. Nevertheless, it has proved challenging to make this next step, and few programs exist that actually provide this level of data feedback.
Currently available cancer quality measures
Despite the definitional complexity of what constitutes quality cancer care, hospitals and providers must ultimately be evaluated and judged as optimal or in need of improvement. To this end, both private and public organizations, including the NQF, Agency for Healthcare Research and Quality, CMS, the Joint Commission, and the American Hospital Association, have been instrumental in motivating the development of more than 100 measures and benchmarks of care specific to cancer. The NQF has perhaps taken the lead by rigorously and thoroughly evaluating measures based on a set of predefined standards, including clinical importance, scientific acceptability, usability, and feasibility of data collection.
NQF
In 2002, the NQF released an initial call for measures to identify opportunities for health care professionals to improve cancer care. This initiative focused on breast, colorectal, and end-of-life cancer care and resulted in 19 NQF-endorsed performance measures. Breast cancer measures related to surgery included post–breast-conserving surgery irradiation, use of adjuvant chemotherapy or hormone therapy, adherence to the College of American Pathologists Breast Cancer Protocol, use of needle biopsy for diagnosis, and evaluation of axillary lymph nodes in early-stage disease. Colorectal cancer measures included adjuvant chemotherapy use, completeness of pathologic reporting, adherence to the College of American Pathologists Colon and Rectum Protocol, and the examination of at least 12 lymph nodes. Because CMS requires NQF endorsement before their use in public reporting or payment incentives, many of these measures are or will soon be implemented at the national scale.
American Society of Clinical Oncology
In a separate program led by the American Society of Clinical Oncology (ASCO) in conjunction with RAND Corporation and Harvard University, health service researchers conducted the National Initiative on Cancer Care Quality study. Findings from this report identified no surgery-specific measures. Nevertheless, treatment-related measures included optimizing chemotherapy dosing, managing side effects, advising patients about treatment options, better documentation of key cancer staging and treatment details, and ensuring that patients at the highest risk of poor outcomes receive the recommended care.
Other Available Measures
Although most measures reflect breast and colorectal cancers, health services researchers have also identified several quality measures in other cancer sites. In a 2009 study focusing on melanoma care, 26 measures were identified as valid according to RAND/UCLA Appropriateness Methodology. Twenty-four of these measures were processes of care and were mainly related to receiving guideline treatments such as ensuring negative margins, examining appropriate numbers of lymph nodes, and undergoing standard preoperative workup algorithms. A separate report focused on developing pancreatic cancer quality indicators that also used RAND/UCLA Appropriateness Methodology. Forty-three indicators were identified as valid, of which 11 were structural, 19 were process related, 4 addressed treatment appropriateness, 4 assessed efficiency, and 5 assessed outcomes.
Gaps in Cancer Quality Measures
Although many measures have been developed, several gaps exist that need to be addressed. First, of the measures currently available, many are not disease specific or focus only on the most common cancers, such as the NQF-endorsed colorectal and breast cancer quality measures. It should be emphasized that although other cancers are less prevalent, they often have even greater variations in quality. For example, with respect to lymph node examinations following cancer surgery, adherence to the 12–lymph node measure is much better in colon cancer than other common cancers such as esophagus, gastric, and pancreas cancers.
Second, there is a lack of surgical outcome assessment in endorsed cancer care quality measures. This is not surprising, given that outcome measures can be considerably more difficult to develop and implement and are at times seen as threatening evaluations. Unlike processes of care, outcomes require the collection of standardized and reliable data for appropriate risk adjustment to account for differences in patient, disease, and procedural risk. Nevertheless, risk-adjusted outcome comparisons may be better reflections of true hospital quality. Recently, the NQF endorsed the first colon resection outcome quality measure, which was developed by the American College of Surgeons (ACS). This measure was demonstrated to be feasible, reliable, and accurate, and, although not intended to be used only after resection for cancer, recent work suggests that cancer-specific variables may not be necessary for robust risk-adjusted hospital quality short-term outcome assessment.
Last, other quality domains that have not been sufficiently addressed include timeliness of care (eg, time from diagnosis to surgery, time for surgical resection to adjuvant chemotherapy in stage III colon cancer) and appropriateness of care (eg, appropriateness of axillary dissection in selected patients with low-risk breast cancer). Nevertheless, these domains are not yet clearly defined, and further research is required before their endorsement and implementation.
Despite the numerous quality indicators currently in existence, without actionable data they cannot affect a meaningful improvement for individual patients with cancer. Nevertheless, it has proved challenging to make this next step, and few programs exist that actually provide this level of data feedback.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree