The primary aim of anal cancer treatment is loco-regional control with preservation of anal function. Phase III trials consistently demonstrate radiotherapy with concurrent 5FU and mitomycin (MMC) chemoradiation is the standard of care for anal cancer. Salvage surgery is associated with considerable morbidity and requires specialised input. With current sophisticated radiological staging and the ability to spare critical normal tissues with intensity-modulated radiotherapy, a “one-size-fits-all” approach is probably inappropriate. Radiotherapy dose-escalation and intensification of the concurrent chemotherapy might improve local control, but may also adversely affect colostomy-free survival. Integration of biologic therapy with conventional chemotherapies looks hopeful in the future.
Key Points
- •
With our current sophisticated radiological staging procedures and ability to spare critical normal tissues with intensity-modulated radiotherapy, a “one-size-fits-all” approach for anal cancer is probably inappropriate.
- •
Radiotherapy dose escalation and intensification of the concurrent chemotherapy might improve local control, but is just as likely to adversely affect colostomy-free survival.
- •
Integration of biologic therapy with conventional chemotherapies looks hopeful.
- •
Patients with anal cancer should be treated from diagnosis by a specialised multidisciplinary team.
Epidemiology and pathogenesis
Incidences and Trends
Anal cancer is an uncommon malignancy with an incidence ranging between 1.0 to 2.5 per 100,000 population in many western countries. In 2011, an estimated 5820 new cases (2140 men and 3680 women) were registered in the United States ; in the United Kingdom, in 2007, there were 790 new cases (292 men and 498 women). The incidence of anal cancer has been increasing over the past 3 decades in Denmark, Sweden and Scotland, most markedly in women. The exception is the United States, where the increases have been greater in men compared with women.
Risk Factors and Etiology
Squamous cell carcinoma (SCC) accounts for more than 90% of anal cancer and is commonly associated with human papilloma virus (HPV) infection. Risk factors include a history of receptive anal intercourse, a history of other HPV-related cancers, human immunodeficiency virus (HIV) infection, immunosuppression after solid organ transplantation, social deprivation, and cigarette smoking. There is no clear association with dietary habits and chronic inflammatory diseases, and the presence of hemorrhoids does not appear to predispose to anal cancer development.
Among men who have sex with men (MSM) in the United States, the incidence of anal cancer is approximately 35 per 100,000. In men who are HIV seropositive, the incidence increases further to 75 to 135 per 100,000. The incidence is also increased among seropositive women (30 per 100,000).
The evidence supporting the role of HPV in the etiology of anal cancer comes from several different lines. A meta-analysis investigated HPV prevalence in anal intraepithelial neoplasia (AIN) grades 1 to 3 and carcinoma from 93 studies conducted in 4 continents and using polymerase chain reaction assays. HPV was identified in 84.5% of cases, paralleling the prevalence seen in cervical and vulval carcinoma in women. In common with cervix cancer, HPV 16 and 18 are the 2 commonest genotypes detected. Prospective data also links HPV seropositivity and risk of subsequent anal and perianal skin cancer. HIV-positive and HIV-negative homosexual men are more likely than the general population to be infected with HPV, often with more than 1 subtype, and are more likely to demonstrate HPV-associated AIN.
Anal Intraepithelial Neoplasia
In a similar manner to cervical carcinogenesis, it is believed that anal cancer arises in most cases through a precursor lesion: AIN. In turn, AIN is graded from 1 to 3 in severity. The prevalence of AIN in the population is generally low and the progression to invasive carcinoma is also low. The prevalence of AIN in HIV-negative homosexual men is high (greater than 36%), however, and almost universal among HIV-positive MSM. In these patients, progression to invasive carcinoma is more likely and is influenced by HIV seropositivity, low CD4 count, and the HPV gentotype.
The natural history from AIN to invasive carcinoma in HIV-positive individuals is also influenced by HIV treatment; namely, highly active antiretroviral therapy. HIV-positive individuals now live longer and have longer exposure to the effects of HPV. Anal SCC is now the commonest malignancy in HIV-positive individuals in the United States.
HPV Vaccination
Prophylactic HPV vaccination against HPV-16 and HPV-18 has been shown to be highly effective in preventing cervical dysplasia and thus cervical cancer. Recently, the quadrivalent HPV vaccine (against HPV types 6, 11, 16, and 18) has also been shown to be highly effective in preventing HPV-16 and HPV-18 associated anal dysplasia, and thus, in theory, may prevent a large proportion of cases of anal SCC in the future.
The role of HPV may have therapeutic implications, as patients with HPV-positive tumors in some anatomic sites appear to be more radiosensitive. This finding may be partly explained because HPV is associated with the proteins E6 and E7, which affect tumor-suppressor proteins p53 and Rb in normal cells, and have apoptotic cellular effects to prevent abnormal cell division.
Epidemiology and pathogenesis
Incidences and Trends
Anal cancer is an uncommon malignancy with an incidence ranging between 1.0 to 2.5 per 100,000 population in many western countries. In 2011, an estimated 5820 new cases (2140 men and 3680 women) were registered in the United States ; in the United Kingdom, in 2007, there were 790 new cases (292 men and 498 women). The incidence of anal cancer has been increasing over the past 3 decades in Denmark, Sweden and Scotland, most markedly in women. The exception is the United States, where the increases have been greater in men compared with women.
Risk Factors and Etiology
Squamous cell carcinoma (SCC) accounts for more than 90% of anal cancer and is commonly associated with human papilloma virus (HPV) infection. Risk factors include a history of receptive anal intercourse, a history of other HPV-related cancers, human immunodeficiency virus (HIV) infection, immunosuppression after solid organ transplantation, social deprivation, and cigarette smoking. There is no clear association with dietary habits and chronic inflammatory diseases, and the presence of hemorrhoids does not appear to predispose to anal cancer development.
Among men who have sex with men (MSM) in the United States, the incidence of anal cancer is approximately 35 per 100,000. In men who are HIV seropositive, the incidence increases further to 75 to 135 per 100,000. The incidence is also increased among seropositive women (30 per 100,000).
The evidence supporting the role of HPV in the etiology of anal cancer comes from several different lines. A meta-analysis investigated HPV prevalence in anal intraepithelial neoplasia (AIN) grades 1 to 3 and carcinoma from 93 studies conducted in 4 continents and using polymerase chain reaction assays. HPV was identified in 84.5% of cases, paralleling the prevalence seen in cervical and vulval carcinoma in women. In common with cervix cancer, HPV 16 and 18 are the 2 commonest genotypes detected. Prospective data also links HPV seropositivity and risk of subsequent anal and perianal skin cancer. HIV-positive and HIV-negative homosexual men are more likely than the general population to be infected with HPV, often with more than 1 subtype, and are more likely to demonstrate HPV-associated AIN.
Anal Intraepithelial Neoplasia
In a similar manner to cervical carcinogenesis, it is believed that anal cancer arises in most cases through a precursor lesion: AIN. In turn, AIN is graded from 1 to 3 in severity. The prevalence of AIN in the population is generally low and the progression to invasive carcinoma is also low. The prevalence of AIN in HIV-negative homosexual men is high (greater than 36%), however, and almost universal among HIV-positive MSM. In these patients, progression to invasive carcinoma is more likely and is influenced by HIV seropositivity, low CD4 count, and the HPV gentotype.
The natural history from AIN to invasive carcinoma in HIV-positive individuals is also influenced by HIV treatment; namely, highly active antiretroviral therapy. HIV-positive individuals now live longer and have longer exposure to the effects of HPV. Anal SCC is now the commonest malignancy in HIV-positive individuals in the United States.
HPV Vaccination
Prophylactic HPV vaccination against HPV-16 and HPV-18 has been shown to be highly effective in preventing cervical dysplasia and thus cervical cancer. Recently, the quadrivalent HPV vaccine (against HPV types 6, 11, 16, and 18) has also been shown to be highly effective in preventing HPV-16 and HPV-18 associated anal dysplasia, and thus, in theory, may prevent a large proportion of cases of anal SCC in the future.
The role of HPV may have therapeutic implications, as patients with HPV-positive tumors in some anatomic sites appear to be more radiosensitive. This finding may be partly explained because HPV is associated with the proteins E6 and E7, which affect tumor-suppressor proteins p53 and Rb in normal cells, and have apoptotic cellular effects to prevent abnormal cell division.
Presentation and diagnosis
Presentation and the Role of the MDT
For UK and European treatment series, mean ages at presentation are between 60 and 70 years; for US series, mean ages are typically a decade earlier, an observation to take into account when comparing outcomes.
Common presenting symptoms are anal pain, bleeding, anal discharge, pruritis ani, and ulceration. Once the anal sphincters are involved, patients complain of discharge and soiling before frank fecal incontinence and tenesmus. In locally advanced disease, perianal infection and fistula formation may occur. Cases may present with enlarged inguinal lymph nodes in the absence of anal symptoms. Clinically palpable (inguinal) lymph nodes occur in 16% to 25% of cases, depending on the clinical setting. Distant metastases at presentation are generally reported as less than 5% in treatment series ; the proportion for all-comer series is unclear.
Increasingly, new cases may present through a variety of other routes: incidental excision of anal tag or hemorrhoidectomy, through AIN surveillance clinics (particularly for high-risk patients), through transplant clinics, and as carcinoma arising within existing perianal Crohn disease (although most of these are mucinous adenocarcinomas rather than SCC).
Within the United Kingdom, each of the 32 cancer networks is required to establish a specialized anal cancer multidisciplinary team (MDT), which meets regularly. The MDT includes a team of colorectal surgeons, clinical oncologists, radiologists, gynecologists, and pathologists, supported by a dedicated MDT coordinator, advanced nurse specialist, and data manager. All patients with a new diagnosis of anal SCC are reviewed in the MDT before initial treatment. In general, no more than 2 consultant surgical core members within the network should perform all salvage surgical operations for anal cancer.
Anatomy and Lymphatic Drainage
For this article, we use the definitions of the anal canal and anal margin used by the National Comprehensive Cancer Network (NCCN). For the anal canal, the superior functional border, separating it from the rectum, has been defined as the palpable upper border of the anal sphincter and puborectalis muscles of the anorectal ring. It is approximately 3 to 5 cm in length, and its inferior border starts at the anal verge, the lowermost edge of the of the sphincter muscles, corresponding to the introitus of the anal orifice. The anal margin starts at the anal verge and includes the perianal skin over a 5-cm to 6-cm radius from the squamous mucocutaneous junction.
The most proximal portion of the anal canal drains to perirectal nodes and nodes along the superior rectal vessels to the inferior mesenteric system, and thence to the para-aortic nodes. There is also drainage to the internal iliac and obturator nodes. The canal above the dentate line drains to internal pudendal nodes, and to the internal iliac system. Venous drainage of the upper half of the canal is mainly by the superior rectal vein to inferior mesenteric vein, whereas drainage of the lower half is by the inferior rectal vein, to the internal pudendal vein and internal iliac vein. Hence, metastases may occur either to liver via the portal system, or lung via the systemic circulation, partly depending on tumor position. The canal below the dentate line drains to the medial group of superficial inguinal nodes with some communication with femoral nodes and to external iliac nodes. Involved nodes are often palpable, but historical pathology studies, using a “clearing” technique, demonstrated that almost half of all involved lymph nodes were smaller than 5 mm in diameter. The inguinal, femoral, and iliac lymph nodes are the most frequent sites for nodal metastases.
Histopathology
Assessment of the integrity of the biopsy specimen should be documented. The size of the tumor in terms of the greatest dimension if possible, and after local excision, the resection margins (specified in mm), both deep and at the periphery, are required to decide if further treatment is advisable. Hence, all the relevant resection margins should ideally be inked.
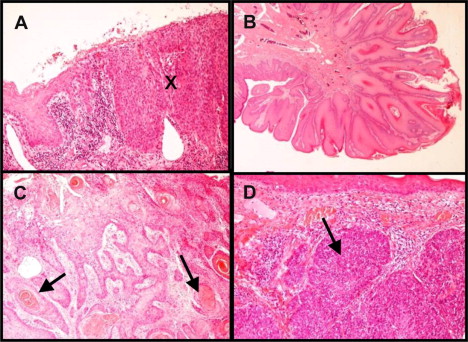
This article does not discuss the diagnosis of non-SCC anal malignancies. Traditionally, SCC has been subdivided into basaloid or cloacogenic types, but these are now recognized as an SCC variant that lacks terminal differentiation; specific prognostic significance is questionable, and does not indicate differences in management ( Fig. 1 ). Verrucous carcinomas are another variant and are sometimes described as giant condylomas or Buschke-Lowenstein tumors, which are often larger but may have a better prognosis than SCC. Grading is subject to considerable interobserver variability, however, and there is considerable heterogeneity in larger tumors. Hence, although high-grade tumors are generally accepted to have a worse prognosis, this has not been confirmed in multivariate analysis from historical surgical series.
Molecular Pathology and Prognostic Factors
Over the past 2 decades, chemoradiation has become the standard treatment (detailed later) and offers an excellent response rate and prognosis for most patients, but there is still considerable heterogeneity as regards their outcomes. Hence, biologic factors/biomarkers that affect outcomes would be useful to provide prognostic information and, in turn, inform individualized therapies.
A recent systematic review examined 29 different biomarkers belonging to 9 different functional classes: tumor suppressors, epidermal growth factor receptor (EGFR), apoptosis regulation, proliferation index, angiogenesis, tumor-specific markers (eg, squamous cell carcinoma antigen and carcinoembryonic antigen), Hedgehog signaling, and telomerase. Thirteen biomarkers were associated with outcome in at least one study, but the tumor-suppressor genes p53 and p21 were the only biomarkers shown to have prognostic value in more than one study. Notably, overexpression of p53 is common in anal carcinomas. Alterations in p53 protein function may result from either mutations in its gene or sequestration by other cellular proteins, such as the E6 viral oncoprotein of the HPV virus. In an analysis of 240 patients randomized in the United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial I (UKCCCR ACT I), the presence of mutated p53 predicted a poorer cause-specific survival. Anal carcinomas appear to have a high proliferative index ; however, only 2 studies have shown its prognostic significance.
In the interpretation of these data, it is important to take residual confounding into account. Thus, for example, some biomarkers may be associated with more advanced disease and may be prognostic Because of coincidence with advanced stage. Prognostic studies are best addressed where treatment is standardized in a narrow stage range.
Staging and initial assessment
TNM Staging
Early trials used the International Union Contra Cancer staging system in which T stage is based on anatomic extent and the proportion of the circumference of the anal canal involved by tumor. Since 2000, studies have used the unified American Joint Committee on Cancer/International Union against Cancer (AJCC/UICC) staging system incorporating primary tumor size (T), lymph node status (N), and distant metastases (M). The seventh edition of the AJCC/UICC classification for anal cancer is shown in Table 1 .
| Primary Tumor (T) | |||
| Tx | Primary tumor cannot be assessed | ||
| Tis | Carcinoma in situ [Bowens disease, high-grade intraepithelial lesion (HSIL), anal intraepithelial neoplasia (AIN) II-III] | ||
| T1 | Tumor smaller than 2 cm in greatest dimension | ||
| T2 | Tumor between 2 cm and 5 cm in greatest dimension | ||
| T3 | Tumor larger than 5 cm in greatest dimension | ||
| T4 | Tumor invading adjacent organs [vagina, urethra, bladder, sacrum] | ||
| Regional lymph nodes (N) | |||
| NX | Regional nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in perirectal nodes | ||
| N2 | Metastasis in unilateral internal iliac and/or inguinal nodes | ||
| N3 | Metastasis in perirectal and/or bilateral internal iliac or inguinal nodes | ||
| Distant Metastasis (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| Anatomic stage/Prognostic groups | |||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| IIIA | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| T4 | N0 | M0 | |
| IIIB | T4 | N1 | M0 |
| Any T | N2 | M0 | |
| IV | Any T | N3 | M0 |
| Any T | Any N | M1 | |
Clinical and Standard Radiological Assessment
Accurate initial clinical staging is clinically important for several reasons :
- 1.
Determination of distant metastases: generally deems the case noncurative;
- 2.
Prognosis and treatment response: in general terms, risk of local disease relapse increases with increasing T size;
- 3.
Gross tumor volume: for planning radiotherapy (RT) delivery;
- 4.
Determination of inguinal node involvement: as inguinal node positivity materially changes the planned RT schedule;
- 5.
Defining follow-up;
- 6.
Defining early anal margin tumors suitable for local excision; and
- 7.
Defining stage for future trial entry.
Clinical examination and digital rectal examination (DRE) remain valuable methods for assessing tumor loco-regional extent. Direct proctoscopy is often difficult in more advanced lesions because of pain. Examination under anesthetic allows more detailed palpation of the anorectal and pelvic structures and may be required for biopsy. Vaginal examination is essential in female patients to assess extension into the postvaginal wall or even breaching of the vaginal mucosa. Clinical assessment of inguino-femoral lymph nodes is helpful to identify palpable nodes, but more accurate assessment of whether there is disease involvement relies of radiological assessment and other techniques.
Although clinical examination is an important initial component of staging assessment, radiological modalities offer additional information and are essential parts of the anal cancer MDT assessment ( Fig. 2 ). Given that definitive chemoradiation is the standard of care, however, there are few data to confirm the performance characteristics of radiological staging, as there can be no histopathological correlation (ie, a lack of a referent standard). Transrectal ultrasound (TRUS) allows a 360° view of the anal canal, and can accurately assess the depth of tumor infiltration and involvement of the sphincter mechanism and fistula tracts. TRUS, however, offers a limited field of view and is poor at assessing lymph node involvement in the mesorectum and pelvis. Magnetic resonance imaging (MRI) provides excellent clarity regarding the primary tumor for RT planning, and offers more accurate information on nodal involvement, particularly in the mesorectum and inguinal regions, than clinical staging with computed tomography (CT). MRI is favored for assessing loco-regional disease extent, as this provides good contrast resolution and multiplanar anatomic detail. The intermediate to high signal intensity tumor is well delineated on T2 and short tau inversion recovery (STIR)-weighted sequences, and primary tumor extent into surrounding structures. TRUS may be superior to MRI for detecting small, superficial tumors; MRI is more effective for N staging. To move to more conformal RT (using intensity-modulated RT [IMRT] or volumetric-modulated arc therapy, detailed later in this article) and run future phase III trials, we need clear definitions of nodal involvement by MRI criteria and subclinical areas, which potentially harbor microscopic disease.
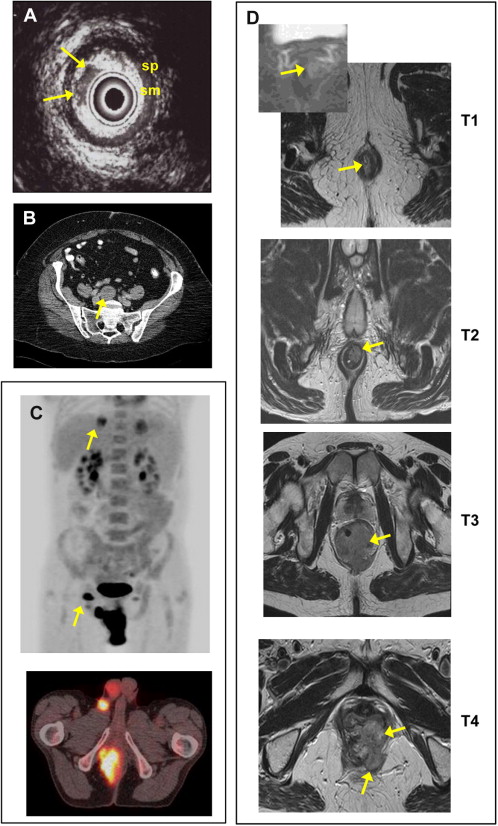
Role of Fluorodeoxyglucose Positron Emission Tomography
Current NCCN treatment guidelines include fluorodeoxyglucose positron emission tomography integrated with CT (FDG-PET/CT) as part of the pretreatment diagnostic workup. FDG-PET/CT can evaluate primary tumor size, lymph node status, and image distant metastases, and can detect uptake in normal-sized but involved nodes with a high positive predictive value, but offers limited accuracy for lymph nodes smaller than 8 mm. As this is a size limit for CT and MRI, a false-negative result is likely to remain false negative in all 3 modalities. PET appears to be highly specific for nodal spread in other pelvic malignancies, such as vulva and cervix. PET/CT can also be used for radiation therapy treatment planning by defining sites of metabolically active tumor. Yet, precise anatomic detail is still poor.
An Italian group examined PET/CT to define stage and assist target volume delineation, and found a change in clinical stage in 18.5% of the 27 patients, leading to a change in treatment intent in 3.7%. The largest UK published series to date found PET/CT changed clinical stage in 23% of patients. The sensitivity of PET was also found to be superior for detection of regional nodal metastases; 89% versus 62% and overall PET changed management in 16% of cases. Others have found that PET/CT complements standard clinical staging information in 10% to 24% of cases, but has little value posttreatment. A recent study from Mount Vernon found that PET/CT diagnosed distant metastatic disease undetected by CT scan in 5%, and altered staging in 42% of patients overall. Currently in the United Kingdom, PET/CT is not part of routine staging and is mainly used to justify radical surgical salvage.
PET/CT should be increasingly used in standard staging protocols. With the advent of advanced planning techniques, such as IMRT, altered RT treatment volumes based on PET/CT imaging may lead to potentially lower toxicity, improved dose accuracy, and a paradigm shift away from therapeutic techniques dependent on the assumption of malignant involvement to those in which we can be more confident of optimal target delineation.
Assessment of Inguino-femoral Nodes
The importance of the accurate prediction of inguino-femoral lymph node involvement is set against the broadly 2 approaches to initial treatment. In the United Kingdom and some European countries, centers follow a 2-phase RT regimen: the phase I dose (typically 30.6 Gy) is a prophylactic treatment of the inguino-femoral and pelvic sidewall lymphatic fields; the phase II dose (to 50.4 Gy) in used in patients with clinically evident nodal disease. The alternative approach is the elective treatment of the inguino-femoral nodes either by RT or surgical nodal dissection.
For early-stage disease (T size ≤3 cm), the risk of inguinal nodal involvement is less than 5%. In contrast, the risk of involvement for clinically staged T3 or T4 may approach 20%. Clinical examination and CT supplemented by fine-needle aspiration cytology (FNAC) has traditionally been the standard of care, but is probably insufficiently accurate. Lymph node metastases in the groin are difficult to assess partly because fewer than 50% of involved lymph nodes will measure less than 5 mm in diameter, and hence, many involved lymph nodes will simply not be palpable or imaged on CT. Reactive nodes in the groins are often enlarged and palpable and may easily be confused with pathologically involved lymph nodes. FNAC of groin nodes with the use of ultrasound may be slightly more accurate, but there are sampling limitations, and negative results do not always give confidence that this is a truly negative result. Excision biopsy, if followed by RT, is likely to lead to added morbidity, such as lymphedema.
More recently, inguinal-femoral node staging by FDG-PET/CT has been advocated, because positive lymph nodes may be more easily identified than with other imaging modalities.
Sentinel lymph node biopsy (SLNB) has been validated in lymph node staging of small breast tumors to spare the need for major axillary dissection. In anal cancer, the rationale is to spare formal inguinal irradiation, but SLNB has yet to fulfill its initial hopes in this setting. There is undoubtedly an important training issue for the surgeon undertaking SLNB, as in one study, 24% of patients had a postoperative complications in the groin. Future trials in this question have to be set against the established observation that the risk of metachronous inguinal node metastasis in patients treated with prophylactic groin radiation is low (4%). SLNB may be useful in the setting of loco-regional recurrence after chemoradiation to decide whether radical inguinal dissection is required at the time of salvage radical anorectal surgery.
Initial treatment
The Evidence for Chemoradiation
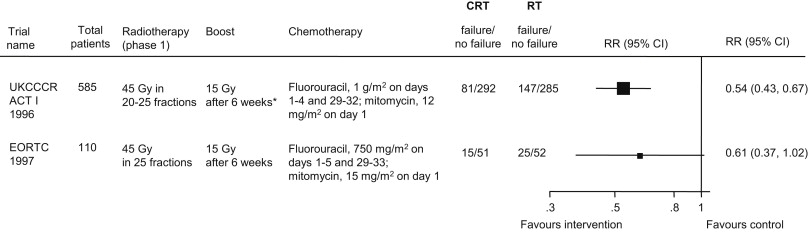
Initial studies of Nigro and colleagues demonstrated high rates of local control (LC) with the use of low doses (approximately 30 Gy) of irradiation with concurrent mitomycin and 5-fluoruracil (MMC/5-FU). Cummings and colleagues reported a series of 190 patients treated with RT alone or chemoradiation (CRT) using 7 sequential regimens and concluded retrospectively improved LC with CRT with the addition of MMC to 5-FU. The definitive evidence for the advantage of CRT is based on 2 phase III trials that compared RT with RT and concurrent MMC/5-FU: the UKCCCR ACT I trial and the European Organization for Research and Treatment of Cancer (EORTC) trial. Both trials showed very comparable results that CRT improves disease-free survival (DFS) compared with RT alone ( Fig. 3 ). By the late 1990s, primary CRT with concurrent MMC/5-FU became standard treatment. This continues today, although a small minority of investigators continue to use external beam RT alone followed by a small volume boost either with photons or electrons (particularly for small T1 tumors).
Interstitial implantation of radioactive sources as a sole modality or as a boost after external beam RT has been used in parts of Europe. Scandinavian retrospective series have described the use of high doses in the region of 60 Gy external beam RT with results that compare favorably with the randomized trials. High rates of LC have been observed in a series from Institute Gustave Roussy of 91% for T1 and T2 tumors, and in a study from San Francisco in patients who were node negative with T1 and T2 tumors had a high 5-year survival rate of 92%. In contrast to this experience in specialist centers, multicenter anal cancer trials demonstrate that even T1 and T2 lesions appear to obtain the benefit from the addition of chemotherapy. Together with sequential phase II studies, these randomized trials have helped to refine techniques of RT and the efficacy of relatively low total radiation doses. However, the optimal schedules, radiation dose, technique, duration of gap, and chemotherapy choice have been repeatedly questioned and remain in some cases the subject of ongoing clinical trials.
Optimizing Chemotherapy Schedules
The Radiation Therapy Oncology Group (RTOG)-8704 trial demonstrated the advantage of adding 2 courses of MMC at a dose of 10 mg/m 2 . With a median dose of 48 Gy and a boost of 9 Gy to histologically confirmed residual disease, the RTOG-8704 confirmed the superiority of MMC/5-FU over 5-FU alone when combined with RT.
The US Intergroup RTOG 98-11 phase III trial randomly assigned 682 patients with anal canal tumors (35% with T3/T4 tumors, and 26% with clinically involved lymph nodes) to either neoadjuvant 5-FU and cisplatin for 2 cycles before concurrent chemoradiation with 5-FU and cisplatin (n = 341), or the standard arm of concurrent chemoradiation with MMC/5-FU (n = 341). The primary end point was 5-year DFS. The neoadjuvant cisplatin-based chemotherapy arm failed to improve DFS, loco-regional control, distant relapse, or overall survival (OS). In fact, trends favored the control arm of mitomycin. Recent presentation of the trial data suggest the outcome for cisplatin could be significantly inferior. By contrast, the requirement for a colostomy was significantly higher in the cisplatin arm compared with the mitomycin arm (19% vs 10%; P = .02). Hematological toxicity was worse with mitomycin. Similar compliance to both chemotherapy and RT in each arm suggests that the differences did not relate to excess toxicity. Both neoadjuvant and concurrent cisplatin was used in the same experimental arm of the trial, however, making analysis of the individual role of each strategy difficult. In total, 10% of patients developed long-term toxicity after CRT, with 5% requiring a colostomy for treatment-related problems.
The Action Clinique Coordonees en Cancerologie Digestive (ACCORD)-03 phase III trial tested 2 cycles of neoadjuvant chemotherapy (NACT) with 5-FU and cisplatin and also radiation dose escalation in a factorial 2 × 2 trial design in 307 patients with SCC cancers larger than 40 mm and/or with pelvic or inguinal involved nodes. The primary end point was colostomy-free survival (CFS). Secondary end points included LC, OS, and cancer-specific survival. The trial compared 45 Gy in 25 daily fractions plus a 15-Gy boost after 3 weeks gap with a higher boost dose of 20 to 25 Gy (ie, 65–70 Gy total dose), but found no benefit in CFS at doses higher than 59 Gy. Toxicity-related dose reductions during CRT were required in 19% of the concomitant cycles. Selected doses of chemotherapy were lower than those prescribed for patients with primary head and neck or esophagus cancer. Newer techniques of irradiation, such as IMRT, should reduce this toxicity by better sparing organs at risk, thereby preventing chemotherapy dose reductions.
Preliminary results of the ACT II multicenter, randomized trial, which recruited 940 patients, have been presented. Patients received 5-FU (1000 mg/m 2 /d on days 1–4, 29–32) and RT (50.4 Gy in 28 daily fractions), and were randomized to receive MMC (12 mg/m 2 , day 1; n = 471) or cisplatin (60 mg/m 2 on day 1, 29; n = 469). A second randomization directed 2 courses of consolidation therapy (n = 448) 5 and 8 weeks after CRT (5-FU/cisplatin, ie, weeks 11, 14), or no consolidation (n = 446). Response was assessed clinically at 11 and 18 weeks, and by CT at 24 weeks. Preliminary results showed almost identical complete response rates (95%) in both arms, with 3-year recurrence-free survival rates of 75% in T1/T2 tumors overall, and 68% for more advanced T3/T4 tumors. Hence, neither strategy, ie, CRT with cisplatin versus CRT with MMC, nor chemotherapy consolidation with cisplatin was more effective for achieving complete clinical response (cCR), reducing tumor relapse or cancer-specific deaths than the standard of MMC/CRT. Acute hematological toxicity was more pronounced for MMC, but nonhematological toxicity similar.
In summary, The RTOG-9811, ACCORD-03, and ACT II phase III trials in anal cancer showed no benefit for cisplatin-based induction and maintenance chemotherapy, or radiation dose escalation above 59 Gy. Neither the RTOG-9208 trial nor the ACCORD-03 trial support the view that radiation dose escalation within a CRT schedule increases LC in anal cancer. The present authors feel that 5-FU and MMC (12 mg/m 2 , day 1) is the recommended standard. A recent update of the RTOG98-11 presented suggests that, with more mature follow-up, there is a significant advantage in 5-year DFS for 5-FU/MMC over induction cisplatin and CRT with 5-FU/cisplatin ( Tables 2 and 3 ).
| Trial Name (Years) | No. of Patients | Design | RT Dose | Testing 1 | Testing 2 | Planned Gap | Primary End Point |
|---|---|---|---|---|---|---|---|
| RTOG 87-04/ECOG (1988–1991) | 291 | 5-FU/RT vs 5-FU/MMC/RT | Phase I 45 Gy–56 Gy median 48 Gy then biopsy of residual disease; 9 Gy boost if + | Addition of MMC 10 mg/m 2 d 1 and 29 to 5-FU-based CRT | 4–6 wk after 45–50.4 Gy if biopsy + | Disease-free survival | |
| RTOG 98-11 (1998–2005) | 644 | NACT cisplatin/5-FU then 5-FU/cisplatin/RT (ie, 4 courses) vs 5FU/MMC/RT | Phase I 45 Gy/25# in 5.0–6.5 wk T3/T4, N+ or residual T2 boost to 54–59 Gy | NACT with 5-FU 1000 mg/m 2 d 1–4, 29–32 and cisplatin 75 mg/m 2 then CRT 5-FU/cisplatin vs MMC 10 mg/m 2 d 1 and 29 and 5-FU 1000 mg/m 2 d 1–4, 29–32 CRT | Max 10-d gap for skin intolerance, but median OTT = 49 d | Disease-free survival | |
| ACT II (UKCCCR) (2001–2008) | 940 | 2 × 2 factorial 5-FU/MMC vs 5-FU cisplatin CRT and consolidation 5-FU/cisplatin vs control | Phase I 30/6 Gy/17# in 3.5 wk then Phase II 19.8 Gy/11# conformal Total 50.4 Gy/28#/38 d no gap | Cisplatin 60 mg/m 2 d 1 and 29 vs 12 mg/m 2 MMC day 1 with 5-FU 1000 mg/m 2 d 1–4, 29–32 CRT | Consolidate 2 courses 5-FU 1000 mg/m 2 and Cisplatin 60 mg/m 2 | No gap; OTT 38 d | Relapse- free survival |
| ACCORD-03 (1999–2005) | 307 | 2 × 2 factorial NACT (5-FU/cisplatin- 2 cycles) vs no NACT Standard vs high-dose boost for responders | Phase I 45 Gy/25#/33 d 3 wk gap then 15-Gy boost standard arms 20 Gy–25 Gy boost (high-dose arms) for responders 40% received brachytherapy boost | NACT with 5-FU 800 mg/m 2 d 1–4, 29–32 and cisplatin 80 g/m 2 on days 1 and 29 then CRT 5-FU/cisplatin on days as above with RT | Dose escalation of RT boost 15-Gy boost standard arms 20 Gy–25 Gy boost (high-dose arms) for responders | 3 wk after 45 Gy CRT completed | Colostomy-free survival Secondary end points included local control, overall survival, and cancer-specific survival. |
| Studies (Authors) | No. of Patients | Median Follow-up | Complete Response | Local Failure Rate | DFS/RFS | Colostomy Rate/Colostomy-Free Survival | Overall Survival |
|---|---|---|---|---|---|---|---|
| RTOG 87-04 (Flam et al, 1996) | 291 | 3 y | Path CR (biopsy) 86% 5-FU 92.2% (MMC) at 4–6 wk post | 16% at 4 y | DFS 51% 5-FU vs 73% with 5-FU/MMC at 4 y | Colostomy rate: 22% with 5-FU vs 9% with 5-FU, MMC; P = .002 Colostomy-free survival: 59% with 5-FU vs 71% with 5-FU/MMC, at 4 y; P = .014 | 71% with 5-FU vs 78.1% with 5-FU/MMC; P = .31 |
| RTOG 98-11 (Ajani et al, 2008) | 644 | 2.5 y | No data on clinical response provided | 25% with 5-FU, MMC vs 33% with 5-FU, cisplatin | DFS 60% with 5-FU, MMC vs 54% 5-FU, cisplatin at 5 y; NS P = .17 | Colostomy rate: 10% with 5-FU, MMC vs 19% with 5-FU, cisplatin; P = .02 at 5 y | 75% with 5-FU, MMC vs 70% with 5-FU, cisplatin; P = .1 at 5 y |
| UKCCCR ACT II (James et al, 2009) | 940 | 36 mo | 94.5% 5-FU/MMC/RT vs 95% 5-FU/Cis/RT at 18 wk, ie, 12 wk post | 11% with MMC; 13% with cisplatin | RFS 75% in both arms at 3 y | Colostomy rate same in both arms (5% with maintenance vs 4% without) | 85% with maintenance at 3 y; 84% without; not significant |
| ACCORD-03 (Peiffert et al, 2012) | 307 | 50 mo | Overall 79% complete clinical response at 2 mo post boost | Arm A 28% Arm B 12% Arm C 16% Arm D 22% overall 19% at 5 y | Tumor-free survival Arm A 64% Arm B 78% Arm C 67% Arm D 62% | 5 y colostomy-free survival Arm A 70% Arm B 82% Arm C 77% Arm D 73% | 5 y specific survival Arm A 77% Arm B 89% Arm C 81% Arm D 76% |
Optimizing RT Dose Schedule
The determination of optimal dose fractionation is limited by a lack of data regarding the pattern of failure. No randomized study has described the site(s) of local failure (within, marginal to, or outside of the RT field). Therefore, it remains uncertain whether most loco-regional failure is attributable to inadequate clinical target volumes, insufficient radiation dose, or intrinsic radioresistance.
Brachytherapy may potentially increase dose to the primary tumor in T3/T4 tumors but requires skill and expertise to avoid radionecrosis owing to an unsatisfactory dose distribution. A low dose-rate iridium interstitial implant was originally advocated as a boost following RT alone after an interval of 6 weeks, but this technique does not achieve current standards of conformal treatment. This delaying strategy influenced the design of the 2 early European phase III studies, in which a brachytherapy boost delivered 25 Gy following CRT after an interval of 6 weeks. Enthusiasm that brachytherapy achieves better outcomes in terms of LC probably reflects patient selection; patients with smaller tumors or good responders to CRT are preferentially selected for brachytherapy boost.
Other Chemotherapy Considerations
Other considerations are as follows ( Table 4 ):
- 1.
All the phase III trials to date used a continuous 4-day or 5-day infusion of 5-FU in the first and fifth weeks of RT. None have used a prolonged venous infusion or an oral fluoropyrimidine during the RT phase, as in rectal cancer. Because the dose and intensity of the chemotherapy administered concurrently with radiation are almost invariably compromised by concerns for acute toxicity, it seems unlikely that such schedules would impact significantly on distant metastases.
- 2.
The central role of MMC in anal cancer therapy is well accepted, yet the dose is still not standardized, and optimal doses are unknown. There is a suggestion that cumulative doses less than 28 mg/m 2 are safe, but higher doses are associated with an increased risk of hemolytic uremic syndrome.
- 3.
The RTOG-8704 trial used an MMC dose of 10 mg/m 2 in weeks 1 and 5. In contrast, the European trials have used a single dose on day 1 of MMC at 12 mg/m 2 (capped at 20 mg total dose in the ACT II study). Although MMC has a rapid systemic elimination, there is little apparent excretion of MMC in urine or feces, suggesting rapid tissue uptake followed by a slow release of the drug from tumor and normal tissues. MMC may remain in hypoxic cells as a radiosensitizer for long periods. It has a recognized biphasic pattern of hematological toxicity. Hence, it is not clear whether a second dose of MMC on day 29 adds to the efficacy of CRT or not, although it clearly would add to toxicity.
- 4.
In addition to the aforementioned phase III trials, 2 European phase II studies, a large retrospective study, and a Brazilian Phase I study have all evaluated cisplatin dosing in concurrent chemotherapy and RT for anal cancer. However, cisplatin doses and schedules vary in all these studies between 60 and 80 mg/m 2 on days 1 and 29, without an obvious advantage to the higher dose. In contrast to clinical studies in cervical cancer, cisplatin has been explored in a weekly schedule in only a single, phase II study.
- 5.
The standard regimen recommended in head and neck cancers is high-dose (100 mg/m 2 ) cisplatin 3-weekly (for 3 cycles) concurrent with RT, and it is felt important to deliver 3 full doses. This high-dose schedule is associated with significant acute and late toxicities, with a significant toxic death rate, and compliance is poor. In contrast, weekly cisplatin at a dose of 40 mg/m 2 is usually well tolerated with acceptable toxicity, in the treatment of nasopharyngeal carcinoma. It seems unlikely that high-dose cisplatin (100 mg/m 2 ) would be deliverable with pelvic RT in anal cancer without excess toxicity, but the optimal dose of 60, 75, or 80 mg/m 2 remains undefined, although 75 mg/m 2 is clearly feasible in cervix cancer.
- 6.
A phase II trial at the M. D. Anderson Cancer Center examined the combination of capecitabine and oxaliplatin with RT. Preliminary results show encouraging response rates of 91% to 100% and CFS of 100%.
- 7.
Carboplatin has also been integrated into chemoradiation schedules. A multicenter randomized study comparing 5-FU/carboplatin with 5-FU/MMC CRT was sponsored by the Societa Italiana Di Proctologia in the 1990s in Italy, but has never been published.
- 8.
Historical reports from Sweden suggest bleomycin was less effective in combination with radiation than 5-FU and mitomycin or 5-FU and carboplatin in more advanced (T3/T4) anal cancer.
- 9.
The EORTC 22011-40014 randomized phase I/II study compared 5-FU and MMC in combination with radiation versus MMC and cisplatin concurrent with radiation. The MMC/cisplatin arm used a cervical cancer schedule (25 mg/m 2 per week) with a total of 175/mg/m 2 . Overall response rate at 16 weeks was 79.5% (31/39) with MMC/5-FU versus 91.9% (34/37) with MMC/cisplatin. With a median follow-up of 2 years, the 1-year progression-free survival was 76.3% in the control versus 94.2% in the MMC/cisplatin arm, and 1-year event-free survival was 74.4% versus 89.2% respectively. Despite these promising results, the schedule was not taken forward into the planned phase III design.