Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
T1
Tumor invades lamina propria or muscular layer
T1a
Tumor invades lamina propria
T1b
Tumor invades muscular layer
T2
Tumor invades perimuscular connective tissue; no extension beyond the serosa or into the liver
T3
Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or another adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts
T4
Tumor invades main portal vein or hepatic artery or invades two or more extrahepatic organs or structures
Regional lymph nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Metastases to nodes along the cystic duct, common bile duct, hepatic artery, and/or portal vein
N2
Metastases to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
Anatomic stage/prognostic groups
Stage 0
Tis
N0
M0
Stage I
T1
N0
M0
Stage II
T2
N0
M0
Stage IIIA
T3
N0
M0
Stage IIIB
T1-3
N1
M0
Stage IVA
T4
N0-1
M0
Stage IVB
Any T
N2
M0
The risk of distant metastases increases as T stage increases. In one review, peritoneal and/or liver metastases were present in 16 %, 42 %, and 79 % of patients with T2, T3, and T4 disease, respectively (Fong et al. 2000). Lymphatic metastases are found in 35–80 % of patients with ≥ T2 disease at diagnosis (Goetze and Paolucci 2008; Tsukada et al. 1996; Bartlett et al. 1996; de Aretxabala et al. 1992; Fong et al. 1998). Due to advanced disease, 15–60 % of patients are candidates for resection at the time of diagnosis (Mekeel and Hemming 2007). Thus, staging prior to considering resection is important and can be accomplished using a combination of preoperative imaging and diagnostic laparoscopy.
12.3.1 Preoperative Imaging
When surgery is being considered in a patient with documented or suspected gallbladder cancer, preoperative imaging is important to identify patients with absolute contraindications to resection. Although preoperative imaging detects extracholecystic disease, it is insensitive for peritoneal disease, which is present in a significant number of patients and requires diagnostic staging laparoscopy for detection Fig. 12.1.
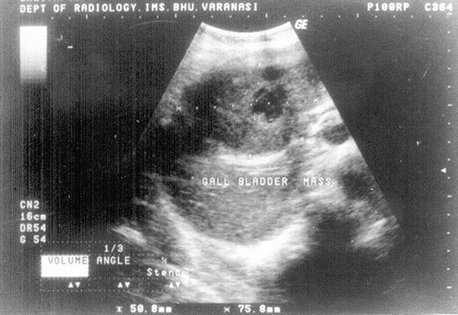

Fig. 12.1
Sonogram shows large heterogeneous mass replacing gallbladder lumen that is consistent with biopsy-proven gallbladder carcinoma
The incidence of patients undergoing nontherapeutic exploratory laparotomy for locally unresectable malignancy has significantly diminished with high-quality, high-resolution preoperative imaging. However, there are no definitive guidelines for imaging prior to surgery (Levy et al. 2001). Cross-sectional imaging can be obtained using multidetector contrast-enhanced computed tomography, contrast-enhanced magnetic resonance (MR) imaging, or MR cholangiopancreatography Fig. 12.2. In selected cases where metastatic disease is suspected, fluorodeoxyglucose-positron emission tomography (FDG-PET) can be used. Most (86 %) of gallbladder cancer is FDG-avid (Corvera et al. 2008; Anderson et al. 2004). In a retrospective study of 93 patients with biliary tract cancer who underwent preoperative FDG-PET scans, the results changed the stage and treatment of 24 % of the patients.
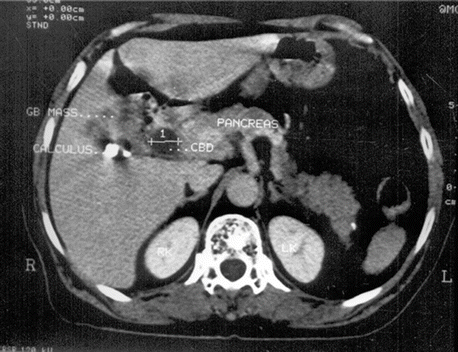

Fig. 12.2
Contrast-enhanced CT scan during hepatic arterial phase shows large carcinoma replacing gallbladder lumen with gallbladder calculus and dilated CBD
12.3.2 Diagnostic Staging Laparoscopy
Diagnostic staging laparoscopy is necessary prior to resection to identify absolute contraindications that may not be apparent on preoperative imaging studies.
Diagnostic staging laparoscopy frequently identifies metastatic disease or other findings that contraindicate tumor resection (Weber et al. 2002; Butte et al. 2011; Gaujoux and Allen 2010). As an example, in a large prospective study of 409 patients undergoing staging laparoscopy for gallbladder cancer, 23 % of patients had disseminated disease (liver surface disease or peritoneal deposits).
Because gallbladder cancer frequently extends directly to adjacent structures such as the liver, stomach, duodenum, pancreas, colon, omentum, or abdominal wall, diagnostic staging laparoscopy may provide valuable information on extent of disease. Laparoscopic ultrasound should be employed as adjunctive imaging to look for satellite lesions in the liver and define the location of the gallbladder tumor, the relationship of the tumor to surrounding blood vessels, and the likelihood of attaining an adequate margin of the liver (Shoup and Fong 2002).
12.4 Contraindications to Resection
Absolute contraindications to surgery for gallbladder cancer include liver metastasis, peritoneal metastases, involvement of N2 nodes (celiac, peripancreatic, periduodenal, or superior mesenteric nodes) and malignant ascites, extensive involvement of the hepatoduodenal ligament, and encasement or occlusion of major vessels. If any of these are confirmed preoperatively on imaging studies, the disease is incurable and surgery should only be considered to palliate specific problems. Direct involvement of the colon, duodenum, or liver does not represent an absolute contraindication. If no contraindications are identified, a diagnostic laparoscopy is performed prior to resection to confirm the absence of peritoneal or metastatic disease (Agarwal et al. 2013).
Patients found to have contraindications to resection based upon preoperative imaging, laparoscopic staging, or intraoperative exploration because of major encasement of vascular structures should be referred for chemotherapy alone or chemoradiotherapy. There is no role for a palliative noncurative radical surgery, for the purpose of debulking, and any subsequent attempts at resection should be undertaken only if it is possible to achieve a complete resection.
Although the value of a debulking simple cholecystectomy has not been definitely proven in this situation, this approach is recommended by some to prevent future episodes of cholecystitis in patients with locally unresectable disease. The optimal way to manage these patients has not been established, and treatment must be individualized based on extent and resectability of the disease and experience of the management team (Shoup and Fong 2002).
12.5 Surgery
Surgery is the only potentially curative therapy (Jayaraman and Jarnagin 2010). Gallbladder cancers that are categorized as stage 0, I, or II (i.e., T stages Tis, T1, or T2) are potentially resectable with curative intent (Table 12.1). Stage T3 tumors are generally locally unresectable due to vascular invasion or involvement of multiple adjacent organs; however, resection may be possible. Stage IVa (T4, N0-1, M0) is occasionally resectable. Stage IVb, M1, or N2 disease represents unresectable disease.
Surgical treatment of gallbladder cancer involves removal of the gallbladder (cholecystectomy) typically with a rim of liver tissue (extended cholecystectomy), except in T1a disease, and may include bile duct resection, lymph node resection, more extensive liver resection, or resection of involved adjacent organs (Foster et al. 2007).
12.5.1 General Approach
Once intra-abdominal metastatic disease has been ruled out with diagnostic laparoscopy, the surgeon can proceed with the resection.
An open rather than laparoscopic procedure is generally recommended (Matsumoto et al. 1992), although some data suggest the feasibility of a planned laparoscopic approach for an early-stage (T1a) gallbladder cancer, the only group for whom the risk of nodal metastases is sufficiently low that more radical resection can be avoided (Table 12.1). However, preoperative staging is not entirely reliable at identifying patients with T1a disease, and improperly staged tumors are at risk for inadequate resection and subsequent recurrence. In a prospective study, the unresectability of disease was not apparent on staging laparoscopy in 75 patients. Among these patients, five patients had surface liver metastases, four had deep parenchymal liver metastases, one had peritoneal deposits, 47 had non-locoregional lymph node involvement, and 18 had locally advanced disease. When a laparoscopic approach has been performed, resection of the port sites is advocated by some groups, but is generally not necessary (Agarwal et al. 2013).
Following cholecystectomy or extended cholecystectomy, the specimen is sent for frozen section to evaluate the margins of the specimen. A more extensive liver resection may be needed to achieve negative margins (e.g., tumors of the gallbladder neck). A frozen section analysis of the cystic duct stump should also be performed. Cystic duct margin status predicts residual disease in the common bile duct. If the cystic duct stump is negative for tumor, a regional lymph node dissection is performed (except T1a tumors). On the other hand, if it is positive, then regional lymphadenectomy plus extrahepatic bile duct resection is undertaken.
For patients in whom the diagnosis of > T1a gallbladder cancer is made incidentally, the status of the cystic duct stump on the pathology report determines the need for lymph node dissection and bile duct resection.
12.5.2 T1 Gallbladder Cancer
Simple cholecystectomy alone is felt to be adequate for patients with tumors that are limited to the lamina propria (T1a). Cure rates following simple cholecystectomy range from 73 % to 100 % in case series. Patients found to have incidental T1a tumors with negative margins are generally felt to be curable with the cholecystectomy that has already been performed. Re-resection for T1a tumors does not appear to provide an overall survival benefit.
Patients with stage T1b disease may benefit from a more radical approach, given that T1b tumors are associated with a higher incidence of lymph node metastases compared with T1a tumors (15 versus 2.5 %) (Ogura et al. 1991, 1994). Some investigators have shown a median survival advantage of over 3 years for extended versus cholecystectomy alone for T1b cancers (9.85 versus 6.42 years, respectively). Extended cholecystectomy (cholecystectomy including a rim of liver tissue) should be performed for medically fit patients who have tumors that invade the muscular layer (T1b) (Table 12.1).
12.5.3 T2 Gallbladder Cancer
Extended cholecystectomy should also be performed in patients with T2 tumor (Wright et al. 2007). The need to perform a more aggressive resection is supported by the high rate of residual disease discovered on re-resection for T2 disease discovered incidentally. In a retrospective study of six major hepatobiliary centers, upon re-resection of T2 tumors found incidentally, residual disease was found in 57 % of the patients at any site; the lymph nodes were involved in 31 %, and the liver was involved in 10 %. Lymph node metastases have been seen in up to 62 % of patients with T2 disease (Shimada et al. 1997). Given the high rate of residual disease seen after re-resection of T2 disease, it is not surprising that high rates of positive margins and high rates of local recurrence (40 %) are found in patients with T2 treated with cholecystectomy alone (Kapoor 2001).
For patients with an incidentally detected T2 tumor on histologic review of the cholecystectomy specimen, re-exploration and extended cholecystectomy are also indicated. Re-exploration identifies residual tumor in 40–76 % of cases, a high likelihood of liver involvement, and nodal metastases with T2 disease. Re-resection significantly increases the likelihood of long-term disease-free survival in patients with T2 disease. In many series, 5-year survival rates increased from 24 % to 40 % to 80 % to 100 % with aggressive surgery.
12.5.4 Resectable T3/T4 or Node-Positive Gallbladder Cancer
In the past, surgeons were reluctant to operate on patients with locally advanced (T3/4) disease because of an overall poor prognosis. Although some series document poor survival even with extended resection (Cubertafond et al. 1999), support for radical surgery in patients with T3 and even T4 disease has increased with the publication of retrospective reports indicating long-term survival in patients with T3 and T4 tumors, 15–63 % and 7–25 % of patients, respectively.
Some groups advocate even more extensive resection involving hepatectomy, pancreaticoduodenectomy, colectomy, and even nephrectomy for patients with higher T stage but potentially resectable disease. Although long-term survivors are reported, morbidity and mortality rates are high (48–54 %, and 15–8 %, respectively).
For patients with regional nodal (N1 disease, limited to cystic, portal, and portacaval nodes) involvement, 5-year survival rates from 28 % to 60 % are reported with radical resection (D’Angelica et al. 2009; Chijiiwa et al. 2000). Results with radical lymphadenectomy are less favorable with N2 disease, particularly if the extent of nodal disease is beyond the hepatoduodenal ligament, posterosuperior pancreaticoduodenal area, and along the common hepatic artery. If preoperative fine needle aspiration confirms involvement of N2 nodes, the patient is not curable; thus, surgery should be performed only for palliation of specific problems.
In appropriately selected patients, the treatment of advanced but resectable gallbladder cancers (T3 disease) results in 5-year survival rates ranging from 28 % to 44 %. In a retrospective study of patients with incidentally discovered gallbladder cancer, patients with T3 disease who underwent re-resection had very high rates of residual disease found in any site (77 %), in lymph node metastases (46 %), and in the liver bed (36 %). Thus, patients with T3 tumors clearly warrant aggressive re-resection.
12.5.5 Controversy over More Aggressive Resections
For patients with gallbladder that extends beyond the mucosa, >T1a, the benefit of more radical surgery has been controversial. Randomized trials comparing simple cholecystectomy with radical surgery for gallbladder cancer have not been performed; all available studies are retrospective series. Some of these series, but not all, link better outcomes with more radical surgery:
In another Japanese series of 1686 resected gallbladder cancers from 172 major hospitals, survival rates were significantly better for patients undergoing radical resection compared with patients with simple cholecystectomy (3-year survival, 66 versus 14 %; 5-year survival, 51 versus 6 %).
At the Mayo Clinic, 22 of 40 patients undergoing potentially curative resection had a simple cholecystectomy, while the remainder had a radical procedure (Chijiiwa et al. 2000). Although 5-year overall survival rates were similar (33 versus 32 %), median survival in patients undergoing radical resection was significantly better (3.6 versus 0.8 years), and for those with transmural extension or nodal metastasis, the only 5-year survivors were those who had undergone extended cholecystectomy.
On the other hand, in a series of 104 patients treated at Memorial Sloan Kettering over a 12-year period, major hepatectomy, resection of adjacent organs other than the liver, and common bile duct excision increased perioperative morbidity and were not associated with better survival. The authors concluded that major hepatic resection (including excision of the common bile duct) was appropriate, when necessary, to clear disease, but not mandatory in all cases.
12.5.6 Managing an Incidental Gallbladder Cancer
In patients undergoing cholecystectomy, unsuspected gallbladder cancer may be diagnosed based upon intraoperative findings or the final pathologic analysis. In three large series combined, incidental gallbladder cancer was found in 31 of 9,497 patients undergoing laparoscopic cholecystectomy (0.33 %). The likelihood of finding a previously unsuspected gallbladder cancer during the course of open cholecystectomy is similar to that of laparoscopic cholecystectomy.
12.5.7 Identified by Pathology
As with patients with gallbladder cancer that is diagnosed preoperatively based upon imaging studies, the management of incidental gallbladder cancer depends upon disease extent (T stage) (Table 12.1).
Most patients for whom a diagnosis of gallbladder cancer is made incidentally on postoperative gallbladder pathology will require a second procedure for curative intent because of a high rate of residual disease. This was illustrated in a series of 439 cases of incidentally found gallbladder cancer from a German registry. Most gallbladder cancers were T2 or higher and positive lymph nodes were found in 21 % and 44 % of the re-resected patients and T2 and T3 tumors. In these cases, re-resection is associated with significantly improved overall survival. Although re-resection is associated with additional risk, improvements in perioperative care and operative technique have decreased perioperative morbidity and mortality associated with major liver surgery in high-volume centers.
12.5.8 Identified Intraoperatively
Since the majority of cases of gallbladder cancer are not diagnosed intraoperatively and, rather, are found incidentally during management of presumed benign gallbladder disease, it is important to have a plan for identifying and managing gallbladder cancer during the course of routine cholecystectomy.
12.6 Resection Techniques
Surgical treatment of gallbladder cancer involves removal of the gallbladder with a margin of the liver (except T1a disease) with or without regional lymph node or common bile duct resection. If frozen section analysis of the cystic duct stump is negative, a regional lymph node dissection is performed; if it is positive, then regional lymphadenectomy is undertaken along with extrahepatic bile duct resection. Resection of adherent involved adjacent organs may be necessary.
12.6.1 Cholecystectomy
Low-stage (Tis, T1a) gallbladder cancers are curable with simple cholecystectomy. Higher-stage tumors will require, at least, extended cholecystectomy. When gallbladder cancer is suspected, an open approach is more often chosen to minimize the risk for bile spillage. Simple cholecystectomy may also be indicated in patients with more advanced disease as a palliative procedure to prevent future episodes of cholecystitis. In patients with locally unresectable disease, however, its value has not been definitely proven in this situation.
12.6.2 Extended Cholecystectomy
For patients in whom a diagnosis of gallbladder cancer (>T1a) has been made preoperatively, the gallbladder and a rim of liver are resected en bloc (extended cholecystectomy), which involves, at a minimum, the removal of at least a 2 cm margin of the liver adjacent to the gallbladder bed. A formal central liver resection may be appropriate depending upon the location of the tumor (the fundus, body, neck).
The margin of the liver to be resected can be scored superficially (around the gallbladder and gallbladder mass) with electrocautery to a depth of a couple of millimeters, or sutures can be placed at each side of the margin of resection with absorbable sutures on a blunt needle. The gallbladder, tumor, and margin of the liver are resected in a single en bloc specimen. Most studies demonstrate the importance of achieving negative margins (R0 resection). Standard techniques for liver resection are used with bleeding minimized by lowering central venous pressure during liver dissection and the use of topical hemostatic agents and surgical hemostatic devices.
The cystic duct and cystic artery are identified by gentle retraction on the infundibulum of the gallbladder, and once it is clearly identified and dissected, each is ligated and divided. A sample of the cystic duct margin should be sent for frozen section. If the cystic duct margin is negative, portal lymphadenectomy should be performed. If the cystic duct margin is positive, bile duct resection (including portal and hepatoduodenal lymphadenectomy) and reconstruction are performed. Additional frozen sections will decide the need for further resection. Intraoperative frozen sections can reliably indicate whether tumor is present, but they cannot reliably predict the depth of tumor invasion (T stage of the tumor) (Donohue et al. 1990; Yamaguchi et al. 1997).
For patients with tumors of the fundus or body of the gallbladder, the tumor is generally far enough from the inflow structures to the liver to allow a margin-negative resection with at least 2 cm nonanatomic wedge resection of the gallbladder fossa or anatomic resection of segments IVb and V (Blumgart 2007). Various resection margins have been proposed, ranging from 1 to 5 cm, and none are solidly based on carefully collected data (Endo et al. 2004). There are no data from randomized trials showing a benefit for anatomic resection of segments IVb and V in patients with localized disease where a negative margin has otherwise been obtained (Sicklick and Choti 2005). Although a nonanatomic resection may provide a sufficient margin, an anatomic approach reduces the risk for bleeding or bile leakage (Scheingraber et al. 2007). The pattern of drainage of the gallbladder veins may provide support for anatomic IVb/V resection over nonanatomic resection. Veins from the gallbladder rarely enter the portal vein; rather, they drain into the middle hepatic vein via the cholecysto-hepatic veins (Misra and Guleria 2006). Direct lymphatic drainage into the liver has also been demonstrated.
Right extended hemihepatectomy should be performed, if possible, for tumors of the body or neck of the gallbladder that involve the right portal triad or a scenario in which the porta hepatis is involved with inflammation and the distinction between tumor and scar is obscured (Bartlett 2000). Right extended hemihepatectomy increases the likelihood of achieving negative margins. In one study evaluating surgical margins, the distance between the front of the carcinoma and the resection plane ranged between 12 and 20 mm after wedge resection, 16 and 35 mm after resections of IVb/V, and 28 and 58 mm after extended hepatic resection (Ogura et al. 1998). Thus, some have advocated routine, right extended hemihepatectomy, or caudate lobe resection, regardless of hepatic involvement for these patients because of the improved likelihood of achieving negative margins and minimizing blood loss by resecting along anatomic planes.
12.6.3 Bile Duct Resection
When the tumor extends into the common bile duct, or frozen section analysis of the cystic duct margin is positive, extrahepatic bile duct resection should be performed. In a study of 115 patients who underwent surgery for gallbladder cancer, 42 % of patients had residual disease in the common bile duct when the cystic duct stump had a positive margin on frozen section. Once a negative common hepatic duct margin is confirmed by frozen section, reconstruction is carried out with a Roux-en-Y hepaticojejunostomy.
Some have advocated routine resection of the extrahepatic bile ducts, regardless of the result of the cystic duct stump frozen section, as a means to achieving a more complete lymphadenectomy (Shimizu et al. 2004). However, several retrospective series have not shown a survival benefit for this approach in the management of gallbladder cancer. Opponents of routine bile duct resection also cite the risk of potential, serious complications of hepaticojejunostomy, such as bile leak and anastomotic stricture. Further supporting this view, a retrospective study found that common duct resection does not necessarily yield a greater lymph node count.
Although the bile ducts do not need to be resected when the cystic duct margin is negative, the ducts may be compromised during skeletonization of the porta hepatis. If the ducts appear ischemic or otherwise injured, resection and reconstruction will become necessary.
Intra-abdominal drains should be placed following resection and reconstruction of the extrahepatic bile ducts if oozing persists or bile leak is anticipated.
12.6.4 Lymph Node Dissection
Lymph node dissection is indicated if frozen section of the cystic duct margin is negative. In many cases, lymph node involvement is not obvious intraoperatively, and thus, even normal appearing nodes should be removed. Lymphatic metastases are found in 35–80 % of patients with gallbladder tumors that invade the perimuscular connective tissue (≥T2).
The extent of the lymphadenectomy is controversial. When lymph node resection is indicated, most experts advocate routine resection of lymph nodes of the porta hepatis and along the hepatoduodenal ligament. When regional node involvement is limited to cystic, portal, and portacaval nodes, 5-year survival rates range from 28 % to 60 % with extended cholecystectomy and lymph node resection. Involvement of the celiac, peripancreatic, periduodenal, or superior mesenteric nodes is associated with significantly worse prognosis with few long-term survivors. Thus, most surgeons do not perform lymphadenectomy beyond the hepatoduodenal ligament.
12.6.5 Laparoscopic Port Site Resection
Although available data suggest that laparoscopic manipulation does not diminish the survival of patients with incidentally found GBC, port site recurrences have been described (Steinert et al. 2006; Whalen et al. 2001). Although some have recommended port site excision at the time of re-exploration, radical resection does not require resection of the previous laparoscopy port sites. If tumor is found in the port sites, this is a marker for peritoneal disease and removal of the port sites will not be curative.
In a study of 113 patients with GBC detected incidentally at laparoscopic cholecystectomy, 69 had port site resection at the time of re-exploration and 44 did not. Port site disease was seen only in patients with T2 or T3 disease and correlated with the development of peritoneal metastases. Port site resection was not associated with overall survival or recurrence-free survival. The authors do not recommend port site resections.
12.7 Palliative Procedures
Patients with unresectable gallbladder cancer may develop jaundice, upper abdominal pain, and symptoms of biliary obstruction. The optimal palliative therapy provides relief of symptoms with minimal perioperative morbidity and mortality. The choice of palliative procedure depends upon the nature of obstructive symptoms and an assessment of medical risk associated with the procedure.
Simple cholecystectomy
Endoscopic or percutaneous biliary drainage
Endoscopic stenting or intestinal bypass
Biliary bypass – Another option in patients who can tolerate surgery is biliary bypass, but many patients fail these procedures with recurrent obstruction as the disease progresses. In one study, intrahepatic segment III cholangiojejunostomy and staying away from the hepatoduodenal ligament, the most common site of disease progression, successfully palliated the majority of patients.
12.7.1 Adjuvant Treatment for Localized, Potentially Resectable Gallbladder Cancer
12.7.1.1 Patterns of Disease Recurrence
In contrast to patients who have margin-positive resections, in whom locoregional recurrences predominate, the pattern of disease recurrence following complete resection of GBC is distant plus local. In a series of 97 patients undergoing surgery for GBC (90 % of whom had a margin-negative resection), isolated locoregional disease as the first site of failure occurred in 15 % of cases, while an initial recurrence involving a distant site, with or without concomitant locoregional recurrence, occurred in 85 % (Jarnagin et al. 2003).
12.8 Benefits of Adjuvant Therapy
12.8.1 Radiation and Chemoradiotherapy
The role of adjuvant radiation therapy (RT) in the treatment of GBC is not well established. Among patients undergoing potentially curative resection, postoperative external beam RT can diminish local recurrence rates, but the lack of randomized trials makes it difficult to ascertain whether survival is favorably impacted. Impressions of a survival advantage have been reported in many retrospective reports in which either RT alone or chemoradiotherapy (generally with a concomitant fluoropyrimidine) was administered (Nakeeb et al. 2002). In most cases, the authors concluded that the patients who underwent RT as a component of therapy (particularly at doses ≥40 Gy) (Cho et al. 2010; Wang et al. 2008) survived longer than those who did not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree