Epithelial ovarian cancer is a peritoneal surface malignancy that most often presents with spread of disease within the peritoneal cavity. Overall 5-year survival is around 50% and progress in improving outcomes is slow. Among other areas of research, hyperthermic intraperitoneal chemotherapy (HIPEC) provides a promising option. This article reviews the current status of treatment of epithelial ovarian cancer, experience with HIPEC to date, and future directions.
- •
Epithelial ovarian cancer (EOC) most often presents with disease that has metastasized. Optimal front-line therapy is associated with median overall 5-year survival of less than 50%.
- •
EOC is a peritoneal surface malignancy that remains within the peritoneal cavity for much of its life history. Recurrence is common, with 70% of cases having peritoneal disease.
- •
The incorporation of hyperthermic intraperitoneal chemotherapy (HIPEC) makes sound theoretic sense at the time of front-line treatment, for consolidation and for the treatment of recurrence.
- •
Studies increasingly report detailed adverse effect and outcome data associated with extensive cytoreductive surgery and HIPEC that allow more informed decision making for the patient and surgeon.
- •
More focused and randomized clinical trials are ongoing that will help to more precisely determine the role of HIPEC in treatment of EOC.
Background
Section Key Points
- 1.
Epithelial ovarian cancer (EOC) affects more than 200,000 women per year worldwide
- 2.
It causes around 125,000 deaths per year worldwide
- 3.
Most often the disease has spread from the ovaries at presentation
- 4.
5-year overall survival (OS) remains at less than 50%
EOC is a peritoneal surface malignancy that remains confined to the peritoneal cavity and retroperitoneal lymph nodes for much of its natural history. Although originally thought to arise from the ovarian surface epithelium it is now considered likely that EOC arises from distal fallopian tube epithelium that becomes attached to the ovary during the time of ovulation. This theory was initially prompted by the discovery of serous tubal intraepithelial carcinomas and occult invasive serous carcinomas, closely resembling ovarian serous carcinoma, in women with a genetic predisposition to ovarian cancer.
Because both primary fallopian tube and primary peritoneal carcinoma are treated in similar fashion as EOC and have similar prognosis, they are all considered as EOC. The percentage contribution of each of these types of EOC is ovary 91.8%, peritoneum 5.3%, and fallopian tube 2.8%.
EOC affects more than 200,000 women annually around the world, causing 125,000 deaths. In the United States it affects more than 22,280 women annually and is responsible for 15,500 deaths. The disease causes few symptoms initially and in most cases has already spread outside the pelvis with 50.2% being in International Federation of Gynecology and Obstetrics (FIGO) stage III (within the peritoneal cavity or involving para-aortic, pelvic, or inguinal lymph nodes) and 13% in FIGO stage IV (beyond the peritoneal cavity, including lung and liver parenchyma).
EOC has a poor overall outcome, with only 46% to 49.7% of women with EOC surviving 5 years. In addition, progress in reducing incidence and mortality has been slow.
Low malignant potential (LMP), sometimes called borderline or atypical, proliferative tumors are a distinct form of EOC that occur at a younger age, at an earlier stage, with a less aggressive behavior, and much better prognosis than invasive EOC. Ten-year relative OS for LMP carcinomas is 95% and, for stage I, II, III, and IV, it is 97%, 90%, 88%, and 69% respectively. They have an overall 5-year survival of 87.3% compared with 49.7% for invasive EOC. LMP tumors are not thought to respond to chemotherapy, although, in those tumors with invasive peritoneal implants or advanced disease, it is sometimes used. There are no data on the use of hyperthermic intraperitoneal chemotherapy (HIPEC) for treatment of LMP tumors and it is not discussed further here. It is important that LMP carcinomas be identified in series reporting HIPEC therapy because their inclusion could skew outcomes.
Current status of treatment of ovarian cancer
Section Key Points
- 1.
The amount of residual disease at the end of cytoreductive surgery is a major prognostic factor in EOC
- 2.
Initial response to platinum chemotherapy defines treatment and prognosis at the time of recurrence
- 3.
Front-line chemotherapy should include a combination of a platinum analogue and a taxane
- 4.
The case for addition of bevacizumab to front-line therapy has not been proven
Discussion about the possible role of HIPEC in the treatment of ovarian cancer must involve an understanding of current standard treatments.
The Natural History Time Points of Ovarian Cancer
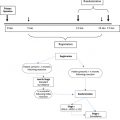
The natural history of EOC can be divided into treatment time points: front-line, front-line failure (persistent disease at the end of front-line treatment), consolidation (maintenance treatment given following a complete response to front-line treatment to reduce the time to recurrence), and recurrent disease. The current status of treatment and role of HIPEC at each time point are considered later.
The Importance of Initial Platinum Response to Prognosis in EOC
Prognosis for patients with EOC is defined by response to platinum and disease can be divided into 2 distinct groups depending on prior response to platinum-containing chemotherapy. Those that are platinum sensitive, recur more than 6 months following a complete response to platinum-containing chemotherapy, whereas those that are platinum resistant, recur less than 6 months following response to treatment. Platinum-resistant tumors also include those that had only a partial response to front-line platinum (persistent disease) or no response (refractory disease).
Front-line Treatment
Standard front-line treatment of EOC involves the combination of cytoreductive surgery (CRS) and chemotherapy. The prognosis for patients with EOC was reported to be related to the amount of residual disease at the end of surgery: patients with the least disease survived longer. This finding has been confirmed in multiple reports since then. Major current debate centers on the extent of CRS that is necessary and whether neoadjuvant chemotherapy (NAC; chemical cytoreduction given before CRS) is of benefit.
Extent of Surgery for EOC
Gynecologic oncology surgeons assess the extent of CRS by the greatest dimension of the largest lesion remaining at the end of CRS. At first, the consensus was that surgery leaving behind disease up to 2 cm in greatest dimension was optimal (as opposed to suboptimal) but over time this has reduced to 1 cm Many gynecologic oncology surgeons currently believe that the goal should be to remove all visible disease.
Surgery Before Chemotherapy or Vice Versa?
Most EOC is inherently sensitive to platinum chemotherapy and it has therefore been used to chemically reduce the volume of ascites and disease before CRS. Three or 4 cycles of a combination of NAC with platinum are considered optimal. Not only can this improve a patient’s performance status and ability to withstand a major surgery but CRS following a response to NAC is often of shorter duration, requires fewer procedures, and has less morbidity with quicker recovery for the patient. It is associated with a higher chance of optimal cytoreduction than initial CRS.
A systematic review of 26 studies including more than 1300 patients undergoing NAC found that the survival outcome was inferior to initial CRS followed by chemotherapy. However, a randomized controlled trial (RCT) by the European Organization for Research and Treatment of Cancer (EORTC), including 632 women with mostly stage IIIC and stage IV disease receiving either initial CRS followed by intravenous (IV) chemotherapy or NAC followed by CRS then further IV chemotherapy, reported similar survival and progression-free survival (PFS) in both arms.
There are proponents of both approaches. Some reserve NAC for those patients with medical comorbidities that would compromise their ability to withstand the stress of major surgery and/or patients with disease unlikely to be amenable to adequate CRS, whereas others use it more liberally.
Front-line Chemotherapy for Advanced EOC
The current primary treatment is front-line CRS followed by platinum and taxane combination chemotherapy. Carboplatin (Paraplatin) has replaced cisplatin (Platinol) as the IV platinum agent because of equal efficacy and reduced toxicity. A standard regimen includes IV carboplatin (dosed by the area under curve of 5–7.5 mg/mL/min) and IV paclitaxel (Taxol) (175 mg/m 2 over 3 hours) repeated every 3 weeks for 6 cycles or IV docetaxel (Taxotere) 75 mg/m 2 over 1 hour plus IV carboplatin AUC 5 over 1 hour every 21 days for 6 cycles provided the disease is responsive. Results from treatment of front-line EOC with CRS followed by chemotherapy are given in Table 1 .
| Residual Disease | Regimen ∗ | Median PFS (mo) | Median OS (mo) |
|---|---|---|---|
| Suboptimal a , | Day 1: paclitaxel 135 mg/m 2 over 24 h Day 1: cisplatin 75 mg/m 2 IV | 14.1 | 26.3 |
| Optimal b , | Day 1: cisplatin 75 mg/m 2 IV at 1 mg/min Day 1: paclitaxel 135 mg/m 2 over 24 h | 19.4 | 48.7 |
| Day 1: carboplatin AUC 7.5 IV Day 1: paclitaxel 175 mg/m 2 IV over 3 h | 20.7 | 57.4 | |
| Optimal b , | Day 1: paclitaxel 135 mg/m 2 IV over 24 h Day 2: cisplatin 75 mg/m 2 IV | 18.3 | 49.7 |
| Day 1: paclitaxel 135 mg/m 2 IV over 24 h Day 2: cisplatin 100 mg/m 2 IP Day 8: paclitaxel 60 mg/m 2 IP | 23.8 | 65.6 |
a Suboptimal, residual disease greater than 2 cm at CRS before chemotherapy.
b Optimal, residual disease less than or equal to 1 cm.Cisplatin (Platinol), carboplatin (Paraplatin), paclitaxel (Taxol), docetaxel (Taxotere).
Role of Normothermic Intraperitoneal Chemotherapy
Gynecologic Oncology Group (GOG) study 172 reported significantly improved PFS and OS for patients treated with a combination of IV and intraperitoneal (IP) chemotherapy following optimal CRS to no greater than 1 cm largest residual lesion size. The median OS for patients treated with combination IV/IP chemotherapy was 65.6 months versus 49.7 months for patients treated with CRS and IV chemotherapy only. A Cochrane Collaboration meta-analysis of all randomized studies using IP therapy for EOC reported a significant survival advantage for IP delivery.
The problem with GOG 172 was that, despite the improved median OS, 65% of patients in the experimental arm experienced recurrence within the follow-up period of the study and only 42% of participants completed all 6 assigned courses of IP chemotherapy. Although many gynecologic oncologists in the United States offer women with small-volume residual disease following initial CRS a modified GOG 172 regimen, IP therapy has not been widely adopted in the oncology community. The reasons for this include the toxicity of the IP chemotherapy and the morbidity and problems associated with IP delivery. The GOG is currently investigating the replacement of cisplatin IP with carboplatin IP.
The 65% recurrence rate in GOG 172 is similar to data reported previously. After a complete pathologic response confirmed at surgery following front-line treatment of stages III and IV EOC, 60% recurred in 5 years and 66% in 10 years. This rate suggests that residual, and initially undetectable, disease is left behind at CRS.
Outcomes After Maximal Front-line CRS in EOC
Despite maximum efforts to resect EOC at surgery, overall 5 year survival for such patients is less than 50%. Eisenkop and colleagues reported a 5 year survival of 49% (median OS of 58.2 months) in 408 patients, most with advanced disease, 98.9% of whom underwent a complete CRS followed by standard chemotherapy. Benedetti-Panici and colleagues reported a 5 year survival of 49.5% (median OS of 62.1 months) for patients with stage IIIB and C or IV treated with maximal CRS and systematic lymphadenectomy. For patients with no visible disease at the end of treatment, median OS may be as high as 106 months.
Results for Latest Trials with Bevacizumab
Although there has been much excitement about studies of bevacizumab in front-line treatment the results have been disappointing and do not support the routine use of this agent. In GOG study 218, 1873 women with stage III (suboptimally debulked or optimally debulked with macroscopic disease) or stage IV disease were randomly assigned to bevacizumab during chemotherapy or, in addition, as consolidation therapy following chemotherapy for 15 months. After a median follow-up of 17.4 months, the addition of bevacizumab as consolidation was associated with a significantly improved median PFS of 14.1 versus 10.3 months with similar median OS (39.7 vs 39.3 months). There was no significant difference in PFS with bevacizumab given at the time of chemotherapy only. Also there was increased toxicity including severe hypertension and gastrointestinal perforation, hemorrhage, or fistula. In another trial, ICON 7, 1528 women with high-risk early stage and advanced stage EOC were randomly assigned to 6 cycles of carboplatin/paclitaxel given every 3 weeks with or without bevacizumab, which was continued for 12 additional cycles. The restricted mean PFS was 21.8 months with bevacizumab versus 20.3 months without ( P = .004). In an updated analysis, PFS (restricted mean) at 42 months was 24.1 months with bevacizumab versus 22.4 months without bevacizumab ( P = .04).
Current status of treatment of ovarian cancer
Section Key Points
- 1.
The amount of residual disease at the end of cytoreductive surgery is a major prognostic factor in EOC
- 2.
Initial response to platinum chemotherapy defines treatment and prognosis at the time of recurrence
- 3.
Front-line chemotherapy should include a combination of a platinum analogue and a taxane
- 4.
The case for addition of bevacizumab to front-line therapy has not been proven
Discussion about the possible role of HIPEC in the treatment of ovarian cancer must involve an understanding of current standard treatments.
The Natural History Time Points of Ovarian Cancer
The natural history of EOC can be divided into treatment time points: front-line, front-line failure (persistent disease at the end of front-line treatment), consolidation (maintenance treatment given following a complete response to front-line treatment to reduce the time to recurrence), and recurrent disease. The current status of treatment and role of HIPEC at each time point are considered later.
The Importance of Initial Platinum Response to Prognosis in EOC
Prognosis for patients with EOC is defined by response to platinum and disease can be divided into 2 distinct groups depending on prior response to platinum-containing chemotherapy. Those that are platinum sensitive, recur more than 6 months following a complete response to platinum-containing chemotherapy, whereas those that are platinum resistant, recur less than 6 months following response to treatment. Platinum-resistant tumors also include those that had only a partial response to front-line platinum (persistent disease) or no response (refractory disease).
Front-line Treatment
Standard front-line treatment of EOC involves the combination of cytoreductive surgery (CRS) and chemotherapy. The prognosis for patients with EOC was reported to be related to the amount of residual disease at the end of surgery: patients with the least disease survived longer. This finding has been confirmed in multiple reports since then. Major current debate centers on the extent of CRS that is necessary and whether neoadjuvant chemotherapy (NAC; chemical cytoreduction given before CRS) is of benefit.
Extent of Surgery for EOC
Gynecologic oncology surgeons assess the extent of CRS by the greatest dimension of the largest lesion remaining at the end of CRS. At first, the consensus was that surgery leaving behind disease up to 2 cm in greatest dimension was optimal (as opposed to suboptimal) but over time this has reduced to 1 cm Many gynecologic oncology surgeons currently believe that the goal should be to remove all visible disease.
Surgery Before Chemotherapy or Vice Versa?
Most EOC is inherently sensitive to platinum chemotherapy and it has therefore been used to chemically reduce the volume of ascites and disease before CRS. Three or 4 cycles of a combination of NAC with platinum are considered optimal. Not only can this improve a patient’s performance status and ability to withstand a major surgery but CRS following a response to NAC is often of shorter duration, requires fewer procedures, and has less morbidity with quicker recovery for the patient. It is associated with a higher chance of optimal cytoreduction than initial CRS.
A systematic review of 26 studies including more than 1300 patients undergoing NAC found that the survival outcome was inferior to initial CRS followed by chemotherapy. However, a randomized controlled trial (RCT) by the European Organization for Research and Treatment of Cancer (EORTC), including 632 women with mostly stage IIIC and stage IV disease receiving either initial CRS followed by intravenous (IV) chemotherapy or NAC followed by CRS then further IV chemotherapy, reported similar survival and progression-free survival (PFS) in both arms.
There are proponents of both approaches. Some reserve NAC for those patients with medical comorbidities that would compromise their ability to withstand the stress of major surgery and/or patients with disease unlikely to be amenable to adequate CRS, whereas others use it more liberally.
Front-line Chemotherapy for Advanced EOC
The current primary treatment is front-line CRS followed by platinum and taxane combination chemotherapy. Carboplatin (Paraplatin) has replaced cisplatin (Platinol) as the IV platinum agent because of equal efficacy and reduced toxicity. A standard regimen includes IV carboplatin (dosed by the area under curve of 5–7.5 mg/mL/min) and IV paclitaxel (Taxol) (175 mg/m 2 over 3 hours) repeated every 3 weeks for 6 cycles or IV docetaxel (Taxotere) 75 mg/m 2 over 1 hour plus IV carboplatin AUC 5 over 1 hour every 21 days for 6 cycles provided the disease is responsive. Results from treatment of front-line EOC with CRS followed by chemotherapy are given in Table 1 .
| Residual Disease | Regimen ∗ | Median PFS (mo) | Median OS (mo) |
|---|---|---|---|
| Suboptimal a , | Day 1: paclitaxel 135 mg/m 2 over 24 h Day 1: cisplatin 75 mg/m 2 IV | 14.1 | 26.3 |
| Optimal b , | Day 1: cisplatin 75 mg/m 2 IV at 1 mg/min Day 1: paclitaxel 135 mg/m 2 over 24 h | 19.4 | 48.7 |
| Day 1: carboplatin AUC 7.5 IV Day 1: paclitaxel 175 mg/m 2 IV over 3 h | 20.7 | 57.4 | |
| Optimal b , | Day 1: paclitaxel 135 mg/m 2 IV over 24 h Day 2: cisplatin 75 mg/m 2 IV | 18.3 | 49.7 |
| Day 1: paclitaxel 135 mg/m 2 IV over 24 h Day 2: cisplatin 100 mg/m 2 IP Day 8: paclitaxel 60 mg/m 2 IP | 23.8 | 65.6 |
a Suboptimal, residual disease greater than 2 cm at CRS before chemotherapy.
b Optimal, residual disease less than or equal to 1 cm.Cisplatin (Platinol), carboplatin (Paraplatin), paclitaxel (Taxol), docetaxel (Taxotere).
Role of Normothermic Intraperitoneal Chemotherapy
Gynecologic Oncology Group (GOG) study 172 reported significantly improved PFS and OS for patients treated with a combination of IV and intraperitoneal (IP) chemotherapy following optimal CRS to no greater than 1 cm largest residual lesion size. The median OS for patients treated with combination IV/IP chemotherapy was 65.6 months versus 49.7 months for patients treated with CRS and IV chemotherapy only. A Cochrane Collaboration meta-analysis of all randomized studies using IP therapy for EOC reported a significant survival advantage for IP delivery.
The problem with GOG 172 was that, despite the improved median OS, 65% of patients in the experimental arm experienced recurrence within the follow-up period of the study and only 42% of participants completed all 6 assigned courses of IP chemotherapy. Although many gynecologic oncologists in the United States offer women with small-volume residual disease following initial CRS a modified GOG 172 regimen, IP therapy has not been widely adopted in the oncology community. The reasons for this include the toxicity of the IP chemotherapy and the morbidity and problems associated with IP delivery. The GOG is currently investigating the replacement of cisplatin IP with carboplatin IP.
The 65% recurrence rate in GOG 172 is similar to data reported previously. After a complete pathologic response confirmed at surgery following front-line treatment of stages III and IV EOC, 60% recurred in 5 years and 66% in 10 years. This rate suggests that residual, and initially undetectable, disease is left behind at CRS.
Outcomes After Maximal Front-line CRS in EOC
Despite maximum efforts to resect EOC at surgery, overall 5 year survival for such patients is less than 50%. Eisenkop and colleagues reported a 5 year survival of 49% (median OS of 58.2 months) in 408 patients, most with advanced disease, 98.9% of whom underwent a complete CRS followed by standard chemotherapy. Benedetti-Panici and colleagues reported a 5 year survival of 49.5% (median OS of 62.1 months) for patients with stage IIIB and C or IV treated with maximal CRS and systematic lymphadenectomy. For patients with no visible disease at the end of treatment, median OS may be as high as 106 months.
Results for Latest Trials with Bevacizumab
Although there has been much excitement about studies of bevacizumab in front-line treatment the results have been disappointing and do not support the routine use of this agent. In GOG study 218, 1873 women with stage III (suboptimally debulked or optimally debulked with macroscopic disease) or stage IV disease were randomly assigned to bevacizumab during chemotherapy or, in addition, as consolidation therapy following chemotherapy for 15 months. After a median follow-up of 17.4 months, the addition of bevacizumab as consolidation was associated with a significantly improved median PFS of 14.1 versus 10.3 months with similar median OS (39.7 vs 39.3 months). There was no significant difference in PFS with bevacizumab given at the time of chemotherapy only. Also there was increased toxicity including severe hypertension and gastrointestinal perforation, hemorrhage, or fistula. In another trial, ICON 7, 1528 women with high-risk early stage and advanced stage EOC were randomly assigned to 6 cycles of carboplatin/paclitaxel given every 3 weeks with or without bevacizumab, which was continued for 12 additional cycles. The restricted mean PFS was 21.8 months with bevacizumab versus 20.3 months without ( P = .004). In an updated analysis, PFS (restricted mean) at 42 months was 24.1 months with bevacizumab versus 22.4 months without bevacizumab ( P = .04).
Current status of HIPEC in front-line ovarian cancer
Section Key Points:
- 1.
The use of HIPEC in EOC makes theoretic sense in view of the high rates of recurrence following standard treatment
- 2.
Experience reported in the literature is increasing
- 3.
There are no RCTs to date
- 4.
HIPEC should ideally be performed on a research protocol
Although the incorporation of HIPEC into the treatment of EOC makes sense at all the natural history time points, there have been no RCTs to compare efficacy against standard treatments. In view of the importance of having minimal tumor volume at the time of HIPEC treatment, the aim of CRS immediately before HIPEC should be to remove all visible disease or at least to achieve a residual lesion(s) size less than or equal to 2.5 mm.
A phase I study including 5 women treated with HIPEC at the time of initial surgery was published in 1999 and since then several nonrandomized studies have been reported in this setting. There are no RCTs and the studies are heterogeneous. Some have no survival data because of their phase I design or because of insufficient follow-up. Outcomes in recent series are given in Table 2 .
| Author | Year | N | PFS | OS | ||
|---|---|---|---|---|---|---|
| Median (mo) | % 5-y Survival | Median (mo) | % 5-y Survival | |||
| Rufian et al | 2006 | 19 | — | — | 38 a | 37 |
| Di Giorgio et al | 2008 | 18 | 25.5 | — | 27 | — |
| Helm et al | 2010 | 26 | 24.8 | 19.7 | 41.7 | 33.3 |
| Deraco et al | 2011 | 26 | 30 | 15.2 | b | 60.7 |
Rufian and colleagues reported on 19 patients with stage III disease treated with paclitaxel for 60 minutes at 41 to 43°C at the time of initial surgery. The mean 5-year OS was 37% but, for those patients with a resection to no macroscopic disease, the median OS was 66 months. In a multi-institutional phase II study, 26 women with stage III to IV EOC were prospectively treated with CRS and closed-abdomen HIPEC with cisplatin and doxorubicin. Following surgery, they received systemic chemotherapy with carboplatin (AUC 6) and paclitaxel (175 mg/m 2 ) for 6 cycles. After a median follow-up of 25 months, 5 year OS was 60.7% and 5 year PFS 15.2% (median 30 months). Excluding a single perioperative death, all patients underwent systemic chemotherapy at a median of 46 days from HIPEC (range 29–75 days).
With the collaboration of 8 centers in the United States, the Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer Registry (HYPERO) of patients treated with CRS and HIPEC was initiated. In the initial report there were 20 patients treated with CRS and HIPEC who fitted entry criteria for GOG 172, having residual disease of less than 1 cm. There was no significant difference in the OS (HIPEC 57.5 months, GOG 172 65.6 months, and 2-year OS 66.4%: 82%) and 2-year PFS (47.6%: 53%).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree