Peritoneal carcinomatosis (PC) carries a worse prognosis than other sites of colorectal metastases. If incomplete resection of PC affords no benefit to patients, complete resection of PC is beneficial in selected patients. The combination of complete cytoreductive surgery to treat the visible PC and hyperthermic intraperitoneal chemotherapy (HIPEC) to treat the nonvisible PC is on the verge of becoming the gold standard. The prognostic impact of a complete resection is high, but that of HIPEC per se is more hypothetical. The presence of a few resectable liver metastases associated with PC is not a contraindication to surgery plus HIPEC.
- •
Peritoneal carcinomatosis (PC) carries a worse prognosis than the usual other sites of colorectal metastases.
- •
If incomplete resection of PC affords no benefit to patients, complete resection of PC is beneficial in selected patients.
- •
The combination of complete cytoreductive surgery to treat the visible PC and hyperthermic intraperitoneal chemotherapy (HIPEC) to treat the nonvisible PC is on the verge of becoming the gold-standard treatment when feasible.
- •
The prognostic impact of a complete resection is high, but that of HIPEC per se is more hypothetical (there is an ongoing trial on this topic).
- •
The presence of a few resectable liver metastases associated with PC is not a contraindication to surgery plus HIPEC.
A reminder of the natural history of colorectal PC
Of patients with metastatic disease at presentation, 5% to 7% exhibit synchronous PC, only one-third have isolated PC, and the disease is technically resectable in only some of these cases of isolated PC. Virtually half of the patients suffering from PC also have liver metastases. Metachronous PC is far more frequent, occurring during cancer progression. However, it is more complex to analyze because it is detected at different stages of the primary cancer and is frequently associated with distant metastases. Some cases of PC are isolated, moderately extended, and accessible to complete cytoreduction.
PC treated with chemotherapy alone: current results
The results of systemic chemotherapy are modest. In 2003, the Amsterdam group published the first randomized trial on HIPEC. It concerned 105 patients with colorectal PC treated between 1988 and 2001, without other metastases and with a good general status, and no signs of diffuse PC. Among them, 51 were randomized to the non-HIPEC group and received systemic chemotherapy. Their median survival was 12.6 months, twice as short ( P = .03) as that observed in the HIPEC group.
Among patients with metastases, the presence of PC as a metastatic site worsens the prognosis: the analysis of the 2095 patients with metastases treated in the North American N9741 and N9841 trials, which compared chemotherapy with FOLFOX (leucovorin/5-fluorouracil/oxaliplatin) or FOLFIRI (leucovorin/5-fluorouracil/irinotecan), showed that median survival was 12.7 months for the 364 patients with PC versus 17.6 months for the 1731 patients without PC ( P <.001). The 5-year survival rate was 4.1% in the first group and 6% in the second group.
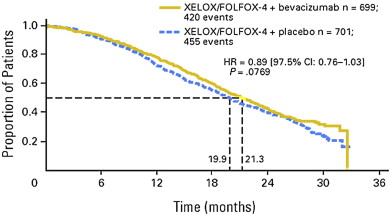
In 2012, the adjunction of targeted molecules such as bevacizumab or cetuximab prolonged median survival by 3 to 6 months. Thus, in the 1401 patients in the randomized study reported by Saltz and colleagues, the median survival of patients receiving FOLFOX + bevacizumab was 21.3 months, compared with 19.9 months ( P = .07) in the patients who received FOLFOX alone ( Fig. 1 ).
Data are emerging on the effectiveness of modern chemotherapy in patients with isolated PC. Klaver and colleagues recently showed in a population-based survival study of 904 patients with primary colorectal cancer who presented specifically with synchronous PC between 1995 and 2008 that PC was the only site of metastasis in 398 patients, and median overall survival was 19 months when patients received targeted therapy such as bevacizumab or cetuximab. This group achieved a higher survival rate than patients presenting with PC plus other metastases, for whom median overall survival was 14 months.
In conclusion, the presence of PC worsens the prognosis of patients with metastases, and currently the median survival of patients with PC is close to 22 months.
PC treated with chemotherapy alone: current results
The results of systemic chemotherapy are modest. In 2003, the Amsterdam group published the first randomized trial on HIPEC. It concerned 105 patients with colorectal PC treated between 1988 and 2001, without other metastases and with a good general status, and no signs of diffuse PC. Among them, 51 were randomized to the non-HIPEC group and received systemic chemotherapy. Their median survival was 12.6 months, twice as short ( P = .03) as that observed in the HIPEC group.
Among patients with metastases, the presence of PC as a metastatic site worsens the prognosis: the analysis of the 2095 patients with metastases treated in the North American N9741 and N9841 trials, which compared chemotherapy with FOLFOX (leucovorin/5-fluorouracil/oxaliplatin) or FOLFIRI (leucovorin/5-fluorouracil/irinotecan), showed that median survival was 12.7 months for the 364 patients with PC versus 17.6 months for the 1731 patients without PC ( P <.001). The 5-year survival rate was 4.1% in the first group and 6% in the second group.
In 2012, the adjunction of targeted molecules such as bevacizumab or cetuximab prolonged median survival by 3 to 6 months. Thus, in the 1401 patients in the randomized study reported by Saltz and colleagues, the median survival of patients receiving FOLFOX + bevacizumab was 21.3 months, compared with 19.9 months ( P = .07) in the patients who received FOLFOX alone ( Fig. 1 ).
Data are emerging on the effectiveness of modern chemotherapy in patients with isolated PC. Klaver and colleagues recently showed in a population-based survival study of 904 patients with primary colorectal cancer who presented specifically with synchronous PC between 1995 and 2008 that PC was the only site of metastasis in 398 patients, and median overall survival was 19 months when patients received targeted therapy such as bevacizumab or cetuximab. This group achieved a higher survival rate than patients presenting with PC plus other metastases, for whom median overall survival was 14 months.
In conclusion, the presence of PC worsens the prognosis of patients with metastases, and currently the median survival of patients with PC is close to 22 months.
PC treated with surgery alone: results
Two situations are possible: either PC is completely resectable or it is not. A clear and wildly accepted definition of resectable PC does not exist. However, one may postulate that when the patient has a relatively good general status and when the extension of PC is limited, resection is technically possible.
When Complete Resection of PC is Not Possible, What are the Results of Incomplete (R2) Resection, and is This Option Beneficial?
In the French study that included 523 patients with colorectal PC operated on between 1990 and 2007 in 23 centers, 84 patients (16%) were not amenable to complete resection of PC, and their median survival was less than 9 months, that is, exactly similar to that obtained with systemic chemotherapy alone during the same period. Recently, the Amsterdam group reported on a series of 43 patients with unresectable PC mainly defined based on the extent of the PC in 6 of the 7 areas of the abdomen, or on involvement of the small bowel. Median survival for the whole group was 6.3 months, but was 9.3 months for the patients who received chemotherapy. Similar results were reported by the Erlangen group: median survival was 8 months (5-year survival: 3%) for the 94 patients who underwent an incomplete (R2) resection of PC, but 15 months (5-year survival: 6%) for the 17 who presented with PC alone.
In conclusion, an incomplete resection of PC does not afford any benefit.
When Complete Resection Alone of PC is Feasible, is it Beneficial?
When one compares the 20 patients who underwent a complete resection of limited PC with the 96 patients who did not undergo a curative resection, as reported by a Japanese group, the 2-year survival rate was 65% in the first group versus 27% in the second ( P = .004). However, it was clear that only patients presenting very limited PC adjacent to the primary were included in the first group.
The Erlangen group recently reported the results of 31 selected patients who underwent complete (R0/R1) resection of their PC that was synchronous with the primary, who were operated on before 2006 (the date they began to use HIPEC). Clearly this option was selected for patients with limited PC and in good general condition. Survival results were unexpectedly good, with a median survival of 25 months and a 5-year survival of 22%. These results were better than those obtained during the same period (before 2006) with systemic chemotherapy (18 months), but probably lower than those obtained with complete resection plus HIPEC. Indeed, the 5-year survival rate after complete surgery plus HIPEC was 48% for patients with minimal peritoneal extension (peritoneal index lower than 10) in the French study that included the learning curves of the 23 participating centers, and 60% for the patients treated by 2 experienced teams reported by Quenet and colleagues.
In conclusion, complete resection of resectable PC is beneficial to patients. The next point will be to prove that it is beneficial to add HIPEC.
PC treated with surgery plus HIPEC: current results
Rationale Behind the Use of HIPEC
This combined treatment associates cytoreductive surgery with HIPEC. The aim of HIPEC is to kill any residual microscopic disease in only one session. A very high concentration of chemotherapy is used together with the effect of hyperthermia. Cultured cancer cells begin to die at 42°C and are totally eradicated at 45°C, explaining why 1°, more or less, is so important in the peritoneal cavity during HIPEC. To change the mean temperature of 43°C in the abdomen during HIPEC to a mean of 42°C divides the efficacy of hyperthermia by 2. Finally, 42° is the minimal temperature required, and a mean temperature of 43° to 44°C is adequate during HIPEC. It is also very important to obtain a high and homogeneous temperature throughout the abdominal cavity. An open procedure is therefore theoretically more efficient than a closed procedure.
Several experimental trials in animals demonstrated the efficacy of this combination, 2 of which are reported in Tables 1 and 2 . Table 1 shows results reported by Koga and colleagues in rats, published as early as 1984, proving the superiority of HIPEC over chemotherapy alone and hyperthermia alone. Table 2 shows similar results published by Pelz and colleagues in 2006.
| Treatment | 37°C | 41.5°C | 42.5°C | Mito 37°C | Mitomycin C + 41.5°C |
|---|---|---|---|---|---|
| Median (d) | 16 | 19 | 51 | 19 | 103 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree