This article focuses on the use of intraperitoneal hyperthermic chemotherapy for the treatment of peritoneal dissemination from appendiceal primary tumors. The first part of the article details patient selection criteria used at the Wake Forest University School of Medicine and the use of preoperative imaging and endoscopic evaluation in the management of this cohort of patients. The second part of the article focuses on clinical outcomes for patients undergoing hyperthermic intraperitoneal perfusion for peritoneal dissemination from appendiceal tumors. Finally, future challenges for the use of hyperthermic intraperitoneal perfusion for appendiceal primary tumors are explored.
- •
The term “pseudomyxoma peritonei” should be used to describe a clinical sign and not a disease. It can be developed by a variety of mucin-producing primaries.
- •
Increased volume of peritoneal disease (PCI >18) is not a contraindication for cytoreduction in low-grade appendiceal primaries.
- •
Increased volume of disease is associated with decreased survival, even in cases of complete cytoreduction. Early diagnosis and treatment is crucial.
- •
Low-grade lesions, including DPAM, may present with positive lymph nodes. The prognosis of this group is decreased and similar to high-grade node-positive appendiceal primaries.
Introduction
Appendiceal cancer is a rare disease with an incidence in the reported literature that varies depending on the histologic types included in the classification of appendiceal malignancies. Historic evidence suggests that appendiceal primaries are diagnosed in approximately 1% of all appendectomy specimens. In a Surveillance, Epidemiology and End-Results database retrospective analysis that excluded low-grade carcinoid tumors, the annual age-adjusted incidence of appendiceal primaries was 0.12 cases per 1,000,000 of population. Appendiceal adenocarcinoma represented 66.5% of these patients. Extrapolating from the fact that the Surveillance, Epidemiology and End-Results program collects data from 14% of the US population, the annual incidence of appendiceal adenocarcinoma in the country should be around 300 to 400 cases, although estimates of up to 2000 cases annually in the United States have been made.
The rarity of the disease has unique implication in diagnosis, classification, terminology, and treatment of these patients. Therefore, several misconceptions are still prevalent among clinicians. First, because most pseudomyxoma peritonei (PMP) cases occur in association with appendiceal primaries the term “pseudomyxoma peritonei” has been used as a synonym to the variant of appendiceal-induced peritoneal surface disease (PSD) that was associated with excessive production of mucin. This is far from accurate given that the term is descriptive and can be applied to any primary (including ovarian, colon, or even pancreatic mucinous) cancer that has the ability to produce mucinous ascites. For the same reason, a diagnosis of PMP lacks prognostic information. Therefore, the term “pseudomyxoma peritonei” is better used as a clinical sign rather than as a distinct disease. Second, not every PMP or appendiceal cancer is associated with long-term survival. Such factors as histologic grade, tumor biology of the primary lesion, age, functional status, and extent of disease at the time of diagnosis determine the prognosis of these patients. Third, not every appendiceal primary is associated with mucin production or ascites. Many patients present with solid peritoneal disease that has no phenotypic difference from any other gastrointestinal malignancy with peritoneal dissemination.
The early symptoms of appendiceal cancer are not disease specific, which in part explains why most of these patients are diagnosed incidentally during surgical exploration or late when peritoneal or systemic dissemination has already occurred. In patients with low-grade tumors, peritoneal dissemination begins with tumor-induced obstruction of the appendiceal lumen. The obstruction in the face of continued mucin production frequently leads to perforation and subsequent dissemination of mucous-producing epithelial cells throughout the peritoneal cavity. The pattern of spread is clearly related to the grade of disease. Low-grade mucin-producing lesions are associated with implantation and spread along the peritoneal surfaces with distant or lymphatic metastases in less than 10% of cases. Dissemination starts in the right lower quadrant of the abdomen, followed by the pelvis, right upper quadrant, then diffusely throughout the peritoneal spaces. This is distinguished from high-grade lesions, which have more substantial risks of systemic metastasis, resulting in poor prognosis. In addition, approximately 7% of low-grade lesions have lymph node involvement and another 16% dedifferentiate into higher-grade lesions during the course of the disease.
The management of patients with PSD from appendiceal neoplasms is still a matter of intense debate. These patients have been traditionally treated with systemic chemotherapy or only debulking procedures. Although low-grade appendiceal tumors treated with debulking procedures can provide some patients with prolonged survival, they are by nature unable to remove all microscopic disease and deal with tumor entrapment in surgical scars. Therefore, more than 90% of these patients inevitably develop local recurrence and death from bowel obstruction. To deal with the problem of postdebulking residual microscopic disease, a variety of “adjuvant” therapies have been evaluated. Intraperitoneal photodynamic therapy, radiation (with 32 P), and chemotherapy instillation have been tried and largely abandoned. In 1980, hyperthermic intraperitoneal chemotherapy (HIPEC) was described. Sugarbaker and coworkers published their early experience in combining cytoreduction with heated intraperitoneal chemotherapy. This combined approach has been more thoroughly evaluated to the point that in many centers, HIPEC is now considered a standard of care in the treatment of peritoneal dissemination from appendiceal cancer.
Systemic chemotherapy for PSD of low-grade appendiceal neoplasms is considered largely ineffective because of the inability of systemically delivered drugs to reach effective intraperitoneal concentrations and the slow kinetics of the malignant cells. Recently, a phase II study in advanced unresectable low-grade appendiceal primaries with concurrent mitomycin C and capecitabine showed a response in 38% of the patients in the form of either stabilization or radiologic reduction in the volume of disease. For high-grade lesions it seems that systemic chemotherapy might improve progression-free survival with the overall survival benefit being derived from the ability to achieve a complete cytoreduction. Chemotherapy with FOLFOX in the neoadjuvant setting for high-grade appendiceal mucinous lesions was related with progression of disease in 50% of patients who had surgical exploration. Tumor response was observed in 29% of the examined specimens. In addition, groups have evaluated several alternative treatment modalities including photodynamic therapy and external-beam radiation therapy. None of these treatments have significantly altered the clinical course of these patients.
Our group’s approach to peritoneal dissemination from appendiceal tumors has been optimal cytoreduction surgery (CRS) with the goal of removal of all gross disease if feasible. This typically entails peritoneal stripping (peritonectomy procedures) and multivisceral resection followed by HIPEC. Selected patients on recurrence undergo repeat exploration and perfusion as dictated by their performance status and symptoms. This article focuses on the use of HIPEC for the treatment of peritoneal dissemination from appendiceal primary tumors. The first part details patient selection criteria used at the Wake Forest University School of Medicine and the use of preoperative imaging and endoscopic evaluation in the management of this cohort of patients. The second part focuses on clinical outcomes for patients undergoing HIPEC for peritoneal dissemination from appendiceal tumors. Finally, future challenges for the use of HIPEC for appendiceal primary tumors are explored.
Preoperative patient evaluation
Patient Selection
Appropriate patient selection is of paramount importance in the management of patients with PSD. All patients presenting to our multidisciplinary clinic have a complete history and physical examination followed by computed tomography (CT) of the chest, abdomen, and pelvis (with oral and intravenous contrast) and tumor markers including CEA, CA19–9, and CA125. All patients undergo pathologic review of previous biopsy or resected tissue.
In general, we use the following eligibility criteria for any patient presenting with documented peritoneal surface malignancy:
- 1.
Patients should be medically fit with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2.
- 2.
There is no extraabdominal disease.
- 3.
The peritoneal disease is resectable.
- 4.
The primary lesion has been resected or is resectable.
- 5.
Parenchymal hepatic metastases if present must be easily resectable.
- 6.
There is no bulky retroperitoneal disease.
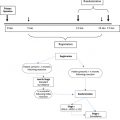
In cases of appendiceal cancer specifically, our selection criteria are modified somewhat based on the grade of the appendiceal primary. For low-grade appendiceal cancer a cytoreduction is attempted regardless of the volume of disease. This is because we recognize that the specific tumor biology is typically indolent and even in cases of incomplete cytoreduction patients receive the benefit of symptomatic control and improved overall survival that can often be measured in years. This is supported by a multi-institutional retrospective review of 2298 patients with PMP where low-grade patients with peritoneal carcinomatosis index (PCI) 31 to 30 had a 10-year survival of 68% when a complete cytoreduction was achieved. The decision to proceed with heated intraperitoneal chemotherapy after incomplete cytoreduction depends on the volume of residual disease and the amount of ascites. In general, patients with voluminous liquid ascites are perfused because their symptoms are effectively controlled at least 75% of the time. In patients without symptomatic ascites but with excessive post-CRS residual disease the perfusion is aborted. In high-grade nonmucinous appendiceal primaries the PCI along with the specific distribution of the peritoneal disease is taken into consideration before proceeding with cytoreduction. Patients with imaging of disease not amenable to complete cytoreduction are not taken into the operating room. These patients are treated with systemic chemotherapy followed by restaging imaging to evaluate for resectability.
Preoperative Imaging
All patients have a contrast-enhanced CT of the thorax, abdomen, and pelvis or magnetic resonance imaging (MRI) within 50 days of the scheduled operation. We prefer CT for low-grade appendiceal malignancies to obtain a rough estimate of the distribution of disease and avoid surprises of possible extra-abdominal involvement. Even though MRI with gadolinium has increased sensitivity in identifying smaller peritoneal implants, we believe that this additional information would not change the clinical decision-making process for low-grade appendiceal patients. In addition, it increases the cost of the preoperative evaluation and patient discomfort.
The strength of the CT is its fundamental ability to detect anatomic details and differences in tissue density. Unfortunately, in peritoneal carcinomatosis one often encounters subcentimeter lesions spread in a carpet fashion. This is consistent with the Netherlands Cancer Institute, which compared intraoperative findings with CT findings and reported CT scan sensitivity between 25% and 37% with a negative predictive value that ranged between 47% and 51%. In the same study the sensitivity for lesions less than 1 cm was between 9% and 24%. In a similar study the false negative rate for the CT to detect small bowel lesions was 60%. Therefore, we explain to our patients that what we see in the preoperative imaging is rarely what we get in the operating room. There is also significant variability among radiologists in the interpretation of the extent of peritoneal carcinomatosis. Therefore, it is imperative for the surgeon who treats PSD to develop expertise in the interpretation of abdominal imaging. Despite thorough preoperative imaging approximately 5% to 10% of patients are deemed not to be operative candidates on exploration.
Conversely, MRI with dilute oral barium and delayed enhanced intravenous gadolinium for mucinous appendiceal lesions when compared with intraoperative findings has been shown to be superior to CT scan in detecting peritoneal metastasis with a sensitivity of 82% to 89%. We use either CT or MRI, but do not routinely obtain both.
Positron emission tomography (PET) imaging has been shown to have decreased sensitivity (10%) for low-volume disease in patients with peritoneal carcinomatosis. The voluminous mucin and low cellular density combined with a low metabolic rate severely limits the use of PET imaging for appendiceal cancers. A retrospective analysis of 33 low- and high-grade appendiceal patients in our institution showed a PET sensitivity of 21% and 8%, respectively. Combing PET with CT increased the sensitivity to 30% and 41%, respectively, for low- and high-grade lesions. PET with or without CT has serious limitations in predicting the extent of carcinomatosis for appendiceal cancer and rarely changes the decision to operate or not on these patients. Therefore, a PET-CT is rarely if ever obtained in our institution.
Colonoscopy for Appendiceal Tumors
We offer colonoscopy in patients with low-grade appendiceal primaries who have not had one in the previous 5 years. This is because we have found that 44% of patients with appendiceal primaries have synchronous colonic polyps. Given the potential of these patients for long survival, we prefer to address a potential need of an additional colonic resection at the same time as CRS-HIPEC. Colonoscopy itself for identification of appendiceal cancer is successful (diagnostic) less than 5% of the time, with most endoscopists finding only a smooth, submucosal cecal mass at the appendiceal orifice with or without free-flowing intraluminal mucin. For high-grade appendiceal tumors, a colonoscopic examination does not commonly alter the course of the disease and is requested in selected patients.
All patients have preoperatively drawn CEA, CA125, and CA19–9. The likelihood of having increased tumor markers has been observed to be equivalent in low- and high-grade lesions. It has been shown that normal preoperative levels of all three are associated with increased likelihood to obtain a complete cytoreduction, probably functioning as a marker of low-volume disease. In addition, normal preoperative CA125 (in male and female patients) has been shown to be associated with prolonged overall survival. Postoperatively, tumor markers are obtained in 3- to 6-month intervals. Levels are taken into consideration along with diagnostic imaging findings and the presence or not of symptoms in evaluating patients for possible recurrence. Patients with voluminous disease and normal preoperative levels rarely benefit from further testing for those markers after HIPEC. A decision to offer a repeat cytoreduction is never based exclusively on the laboratory tumor marker values.
Preoperative patient evaluation
Patient Selection
Appropriate patient selection is of paramount importance in the management of patients with PSD. All patients presenting to our multidisciplinary clinic have a complete history and physical examination followed by computed tomography (CT) of the chest, abdomen, and pelvis (with oral and intravenous contrast) and tumor markers including CEA, CA19–9, and CA125. All patients undergo pathologic review of previous biopsy or resected tissue.
In general, we use the following eligibility criteria for any patient presenting with documented peritoneal surface malignancy:
- 1.
Patients should be medically fit with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2.
- 2.
There is no extraabdominal disease.
- 3.
The peritoneal disease is resectable.
- 4.
The primary lesion has been resected or is resectable.
- 5.
Parenchymal hepatic metastases if present must be easily resectable.
- 6.
There is no bulky retroperitoneal disease.
In cases of appendiceal cancer specifically, our selection criteria are modified somewhat based on the grade of the appendiceal primary. For low-grade appendiceal cancer a cytoreduction is attempted regardless of the volume of disease. This is because we recognize that the specific tumor biology is typically indolent and even in cases of incomplete cytoreduction patients receive the benefit of symptomatic control and improved overall survival that can often be measured in years. This is supported by a multi-institutional retrospective review of 2298 patients with PMP where low-grade patients with peritoneal carcinomatosis index (PCI) 31 to 30 had a 10-year survival of 68% when a complete cytoreduction was achieved. The decision to proceed with heated intraperitoneal chemotherapy after incomplete cytoreduction depends on the volume of residual disease and the amount of ascites. In general, patients with voluminous liquid ascites are perfused because their symptoms are effectively controlled at least 75% of the time. In patients without symptomatic ascites but with excessive post-CRS residual disease the perfusion is aborted. In high-grade nonmucinous appendiceal primaries the PCI along with the specific distribution of the peritoneal disease is taken into consideration before proceeding with cytoreduction. Patients with imaging of disease not amenable to complete cytoreduction are not taken into the operating room. These patients are treated with systemic chemotherapy followed by restaging imaging to evaluate for resectability.
Preoperative Imaging
All patients have a contrast-enhanced CT of the thorax, abdomen, and pelvis or magnetic resonance imaging (MRI) within 50 days of the scheduled operation. We prefer CT for low-grade appendiceal malignancies to obtain a rough estimate of the distribution of disease and avoid surprises of possible extra-abdominal involvement. Even though MRI with gadolinium has increased sensitivity in identifying smaller peritoneal implants, we believe that this additional information would not change the clinical decision-making process for low-grade appendiceal patients. In addition, it increases the cost of the preoperative evaluation and patient discomfort.
The strength of the CT is its fundamental ability to detect anatomic details and differences in tissue density. Unfortunately, in peritoneal carcinomatosis one often encounters subcentimeter lesions spread in a carpet fashion. This is consistent with the Netherlands Cancer Institute, which compared intraoperative findings with CT findings and reported CT scan sensitivity between 25% and 37% with a negative predictive value that ranged between 47% and 51%. In the same study the sensitivity for lesions less than 1 cm was between 9% and 24%. In a similar study the false negative rate for the CT to detect small bowel lesions was 60%. Therefore, we explain to our patients that what we see in the preoperative imaging is rarely what we get in the operating room. There is also significant variability among radiologists in the interpretation of the extent of peritoneal carcinomatosis. Therefore, it is imperative for the surgeon who treats PSD to develop expertise in the interpretation of abdominal imaging. Despite thorough preoperative imaging approximately 5% to 10% of patients are deemed not to be operative candidates on exploration.
Conversely, MRI with dilute oral barium and delayed enhanced intravenous gadolinium for mucinous appendiceal lesions when compared with intraoperative findings has been shown to be superior to CT scan in detecting peritoneal metastasis with a sensitivity of 82% to 89%. We use either CT or MRI, but do not routinely obtain both.
Positron emission tomography (PET) imaging has been shown to have decreased sensitivity (10%) for low-volume disease in patients with peritoneal carcinomatosis. The voluminous mucin and low cellular density combined with a low metabolic rate severely limits the use of PET imaging for appendiceal cancers. A retrospective analysis of 33 low- and high-grade appendiceal patients in our institution showed a PET sensitivity of 21% and 8%, respectively. Combing PET with CT increased the sensitivity to 30% and 41%, respectively, for low- and high-grade lesions. PET with or without CT has serious limitations in predicting the extent of carcinomatosis for appendiceal cancer and rarely changes the decision to operate or not on these patients. Therefore, a PET-CT is rarely if ever obtained in our institution.
Colonoscopy for Appendiceal Tumors
We offer colonoscopy in patients with low-grade appendiceal primaries who have not had one in the previous 5 years. This is because we have found that 44% of patients with appendiceal primaries have synchronous colonic polyps. Given the potential of these patients for long survival, we prefer to address a potential need of an additional colonic resection at the same time as CRS-HIPEC. Colonoscopy itself for identification of appendiceal cancer is successful (diagnostic) less than 5% of the time, with most endoscopists finding only a smooth, submucosal cecal mass at the appendiceal orifice with or without free-flowing intraluminal mucin. For high-grade appendiceal tumors, a colonoscopic examination does not commonly alter the course of the disease and is requested in selected patients.
All patients have preoperatively drawn CEA, CA125, and CA19–9. The likelihood of having increased tumor markers has been observed to be equivalent in low- and high-grade lesions. It has been shown that normal preoperative levels of all three are associated with increased likelihood to obtain a complete cytoreduction, probably functioning as a marker of low-volume disease. In addition, normal preoperative CA125 (in male and female patients) has been shown to be associated with prolonged overall survival. Postoperatively, tumor markers are obtained in 3- to 6-month intervals. Levels are taken into consideration along with diagnostic imaging findings and the presence or not of symptoms in evaluating patients for possible recurrence. Patients with voluminous disease and normal preoperative levels rarely benefit from further testing for those markers after HIPEC. A decision to offer a repeat cytoreduction is never based exclusively on the laboratory tumor marker values.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree