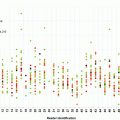
Fig. 2.1
Incidence/mortality of CRC and screening uptake rates over time. CRC incidence/mortality reported as rates among adults age 50 or older (Surveillance, Epidemiology and End Results database) to reflect the screening population. To cover the entire timeline, CRC screening test uptake rates derived from the National Health Information Survey were used for data before 1992 and Behavioral Risk Factor Surveillance System data were used for 1997–2010. The surveys did not differentiate between flexible sigmoidoscopy and colonoscopy for lower endoscopy; however, the sharp increase in lower endoscopy since 1995 is almost entirely the result of an increased use of colonoscopy.CRC colorectal cancer. (Adapted from Ref. [107]. With permission from Elsevier)
This chapter presents the historical basis for CRC screening , reviews the data available regarding current screening options for individuals at average and increased CRC risk, and reviews recommendations for surveillance after polyp removal; when possible, US and international guidelines will be compared. A detailed discussion of familial CRC syndromes or CRC associated with inflammatory bowel disease is outside the scope of this chapter; however, these topics are reviewed in detail elsewhere [4–6].
History and Rationale for CRC Screening
In the USA, screening for CRC has been promoted since the mid-1970s but the tools that are currently used for screening have a much longer history. Although the Corpus Hippocraticum, dating back to the fourth and fifth centuries BC, recorded the first rudimentary attempt at endoscopy with a rectal speculum, most historians credit Philipp Bozzini (Fig. 2.2a) as the creator of the first “modern” endoscope in 1806—the Lichtleiter or light conductor (Fig. 2.2b). The device was constructed with double aluminum tubes (to be inserted in the body orifice being examined) and angled mirrors to project internal structures to the human eye, employing a single candle as a light source [7]. Rigid sigmoidoscopes have long been used diagnostically and screening sigmoidoscopy was developed in the 1950s [8].
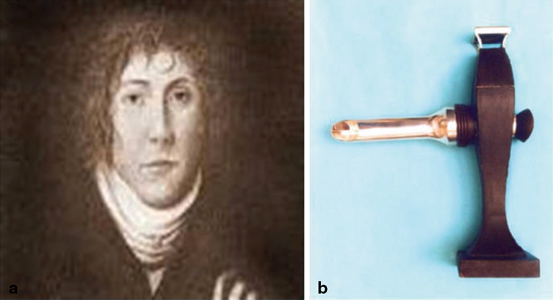

Fig. 2.2
a Philipp Bozzini (1773–1809) is credited with developing the first “modern” endoscope. b The Lichtleiter or light conductor. (Adapted from Ref. [108]. With permission from Nature Publishing Ltd.)
Fecal occult blood testing (FOBT) also has a long history. In the 1850s, Christian Friedrich Schonbein first recognized the chemical reaction causing rapid bluing of guaiac (a resin from the West Indian gouyaca plant) when exposed to ozonized air [9]. Guaiac contains a phenolic compound that is oxidized to a quinone by hydrogen peroxide in a reaction catalyzed by peroxidases including hemoglobin. Von Deen developed a guaiac-based test for occult blood in 1863 [10]. Greegor stimulated widespread interest in FOBT when he reported, in 1967, that asymptomatic CRC could be detected by the presence of blood in the stool [11]. The immunologic tests to detect human hemoglobin were introduced in the 1970s [12], commercialized in the 1980s, and are now considered preferable to standard guaiac-based FOBT because of better performance characteristics (see below). Several FIT tests have now been approved by the US Food and Drug Administration (US-FDA).
As early as 1977, the American Cancer Society (ACS) recommended CRC screening with digital rectal exam and rigid proctoscopy as part of a cancer-related health checkup [13]. The rationale for screening was largely based on observations that patients with screen-detected CRCs had earlier stage disease and longer survival than those with symptomatic CRCs. Compelling evidence of the effectiveness of CRC screening emerged with the completion of large randomized screening trials beginning in the 1990s. On the basis of these trials, the US Preventive Services Task Force (USPSTF) initially recommended CRC screening with annual FOBT and/or sigmoidoscopy in 1995 with a grade B recommendation citing fair evidence of effectiveness [14]. In 2002, the USPSTF upgraded CRC screening to a grade A recommendation stating that the USPSTF “strongly recommends that clinicians screen men and women aged 50 and older who are at average risk for colorectal cancer.”In 2004,CRC screening became a Healthcare Effectiveness Data and Information Set (HEDIS) performance measure, essentially establishing that CRC screening is an accepted standard of care in the USA (HEDIS measures are used by more than 90 % of US health plans to measure performance). CRC screening guidelines in the USA have evolved over time (Fig. 2.3), largely based on the results of the trials that are described in this chapter.
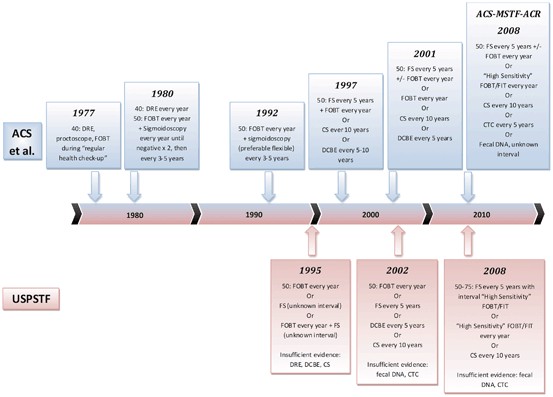

Fig. 2.3
Timeline of US colorectal cancer screening guidelines. ACS guidelines changed to ACS-MSTF-ACR guidelines in 2008. Prior to 2008, MSTF published independent guidelines [14, 35, 59, 109–111].ACS American Cancer Society, ACS-MSTF-ACR American Cancer Society-Multi Society Task Force-American College of Radiology. (Adapted from Ref. [108]. With permission from Springer Verlag)
Average-Risk Screening Options
Current CRC screening options can be categorized into stool-based testing and structural radiographic or endoscopic imaging. Stool-based tests detect the consequences of colonic neoplasia (bleeding or shedding of neoplastic cells into the stool) and, as a one-time test, are better at detecting cancers than precancerous colonic polyps, while imaging modalities (endoscopy, radiology) can directly visualize both colonic polyps and cancers. The advantages, disadvantages, and performance characteristics of these tests are compared in Table 2.1.
Table 2.1
Summary of colorectal cancer Screening Modalities (Adapted from Ref. [108]. With permission from Springer Verlag)
Test | Advantages | Disadvantages | Sensitivity | Specificity | Screening interval | Guideline support | Cost per test |
|---|---|---|---|---|---|---|---|
Imaging tests | |||||||
CS | Can visualize entire colon Performed every 10 years Can remove/biopsy lesions Can diagnose other diseases Single-step diagnostic and treatment Minimal patient discomfort | Invasive Sedation required, patient must be accompanied Time-consuming Expensive Full bowel preparation required Risk of bleeding, perforation Operator dependent, bowel preparation dependent | Generally considered “gold standard” 90 % (when using CTC as standard) for adenoma 5 mm, 97 % for advanced adenomaa | Generally considered “gold standard” | 10 years | ACG ACS-MSTF-ACR USPSTF ASGE WGO | US$ > 1000c |
FS | Simpler bowel preparation than CS Sedation not required Quick Performed every 5 years Does not require specialist or physician Lower risk than CS | Does not visualize entire colon Patient discomfort Risk of bleeding, perforation Two-step test Operator dependent, bowel preparation dependent | 60–70 % for “clinically significant neoplasia”b | Equivalent to CS for region visualized | 5 years | ACG ACS-MSTF-ACR USPSTF ASGE WGO CCA/CAN | US$150–300c |
DCBE | Can visualize entire colon No sedation required Performed every 5 years Lower risk than CS | Insensitive for lesions 1 cm Less training for technicians/radiologists administering and interpreting exam Full bowel preparation required Two-step test | 50 % for adenomas 1 cm 39 % for all polyps | 96 % for adenomas > 10 mm | 5 years | ACS-MSTF-ACR | US$300–400c |
CTC | Can visualize entire colon Less time consuming than endoscopy No sedation required Performed every 5 years Lower risk than CS | Can miss polpys 1 cm Full bowel preparation required Unclear how to follow extra-colonic findings Expensive Two-step test Radiation exposure Variability in performance | 6–9 mm: 23–86 % / = 10 mm: 52–92 % | 86–95 % | 5 years | ACG ACS-MSTF-ACR USPSTF WGO | US $ > 1000c |
Stool-based tests | |||||||
gFOBT | Low risk, noninvasive Widely available No bowel preparation Inexpensive Home testing | High false-positive rate Insensitive for adenomatous lesions Requires frequent testing Two-step test Pretest dietary limitations | For CRC: Single test: 30 % Multiple nonrehydrated: 50–60 % Multiple rehydrated: 80–90 % | For CRC: 87–98 % | 1 year | ACG ACS-MSTF-ACR USPSTF ASGE EU WGO CCA/CAN BSG/ACPGBI | US$ 13 [115] |
FIT/iFOBT | Low risk, noninvasive Widely available No bowel preparation Inexpensive Home testing No dietary restrictions More specific to lower GI bleeding Detects human globin | High false-positive rate Insensitive for adenomatous lesions Requires frequent testing Two-step test | 81.9–94.1 % for CRC 25–27 % for advanced adenoma 67 % for “clinically significant neoplasia” | 87.5 % for CRC97–93 % for advanced adenoma 91.4 % for “clinically significant neoplasia” | 1 year | ACG ACS-MSTF-ACR USPSTF ASGE WGO CCA/CAN BSG/ACPGBI | US$ 20 [115] |
Stool DNA | Low risk, noninvasive No bowel preparation Home testing No dietary restrictions Higher sensitivity than other stool tests | Need to collect an entire stool sample More expensive than other stool tests Unclear how to manage false-positive results Uncertain surveillance interval Two-step test | 25–51 % for CRC 20–41 % for advanced adenomas+CRC | 94–96 % for CRC | Unknown ACG recommends 3 years | ACG ACS-MSTF-ACR WGO | US$ 350c |
Stool-Based Tests
Stool-based CRC screening tests include guaiac-based and immunochemical FOBTs, and more recently, stool DNA tests. The concept of stool testing is based on the observation that colonic neoplasms can both bleed and shed cells into the stool. FOBT is the most widely used CRC screening modality in the world [15] and has been the most rigorously evaluated (see Table 2.2).
Table 2.2
Summary of randomized controlled trials for fecal occult blood testing (FOBT) (Adapted from Ref. [108]. With permission from Springer Verlag)
Trial | Screening | Follow-up (years) | Testing | Participants | Attendance (first screen||at least 1||subsequent rounds) | Sensitivitya | PPVb CRC | PPV adenoma | CRC incidence | CRC mortalityc | All-cause mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|
Minnesota | Annual (A) and biennial (B) | 18 | Hemoccult Rehydrated | 46,551 | – || 75 % ann, 78 % bi || – | 92.2 % | 0.9–6.1 % | 6–11 % | A: 0.8 (0.73–0.94, p 0.001) B: 0.83 (0.73–0.94, p = 0.002) | A: 0.67 (0.51–0.83, p 0.05) B: 0.79, 0.62–0.97, p 0.05) | 342 (334–350)d A: 340 (333–348) B: 343 (336–351) NS |
Nottingham [24] | Biennial | 11.7 | Hemoccult Not rehydrated | 152,303 | 53.4 % || 59.6 % || – | 57.2 % | 9.9–17.1 % | 42.8–54.5 % | 1.51 versus 1.53/1000 person yr NS | 0.87 (0.78–0.97, p = 0.010) | 1.01 (0.96–1.05) NS |
Funen [21] | Biennial | 17 | Hemoccult II Not rehydrated | 61,939 | 66.8 % || – || 91–94 % | 55 % | 5.2–18.7 % | 14.6–38.3 % | 1.02 (0.93–1.12) NS | 0.84 (0.73–0.96, p < 0.05) | 0.99 (0.97–1.02) NS |
Goteborg [25] | Bienniale | 15.75 | Hemoccult II Rehydrated | 23,916 | 63 % || 70 % || 60 % | 82 % | 4.8 % | 14 % | 0.96 (0.86–1.06) | 0.84 (0.71–0.99, p < 0.05) | 1.02 (0.99–1.06) NS |
Guaiac FOBT
Guaiac-based tests detect heme in the stool by the presence of a peroxidase reaction, which turns the guaiac-impregnated paper blue. Most screening protocols require collecting stool samples from three consecutive bowel movements at home to optimize sensitivity. Patients are typically instructed to avoid aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) for 7 days and vitamin C, red meat, poultry, fish, and raw vegetables for 3 days prior to testing to improve specificity. However, a systematic review indicated that a recommended restricted diet did not decrease FOBT false-positivity rates, but did decrease compliance to testing [16]. There are a variety of commercial FOBTs available. The initial tests such as Hemoccult and Hemoccult-II have been shown to be effective in large prospective screening trials (Table 2.2) and are the standard by which subsequent FOBTs have been compared, but they have substantially lower sensitivity for CRC than Hemoccult SENSA (see below).
Performance Characteristics
The performance characteristics of guaiac FOBTs (gFOBTs) can be assessed as a one-time test for the detection of CRC or adenomas of the colon but gFOBTs are recommended to be repeated every 1–2 years, so the performance of a program of gFOBT testing is also important (this distinction highlights the critical importance of ongoing compliance as an issue for stool testing). The performance characteristics of gFOBTs vary with the prevalence of CRC and the age of the population screened and there is even greater performance variability among the types of gFOBTs [17–21]. Of all of the commercial kits available, Hemoccult SENSA has the highest one-time sensitivity for CRC (64–80 %), but a lower specificity (87–90 %) than the other gFOBTs (sensitivity < 50 %; specificity ~ 95 %) [22].
Efficacy
The clinical efficacy of FOBT has been established by controlled trials using the lower sensitivity Hemoccult or Hemoccult II tests. The first trial, reported by Mandel et al. [23] from the University of Minnesota, randomized 46,551 patients to annual FOBT, biennial FOBT, or a control arm. Mortality due to CRC was decreased by 33 % at 13 years in the annual screening group and 21 % at 18 years in the biennial screening group when compared to the control arm. Subsequently, three European trials also demonstrated a CRC mortality benefit ranging from 13 to 16 % using biennial screening [21, 24, 25]. Longer-term follow-up of the US study showed that gFOBT screening led to a 17–20 % lower incidence of CRC [23] (Table 2.2) and that the mortality benefits have been maintained through 30 years of follow-up [26].
There is no direct evidence that screening with FOBT decreases all-cause mortality (Table 2.2). Although none of the trials were powered to assess an effect on all-cause mortality, a meta-analysis of the three major controlled trials [27] found that screening was associated with a significant decrease in CRC mortality, a significant increase in non-CRC mortality and no impact on overall mortality.
Fecal Immunochemical Tests
Immunochemical tests for blood in the stool have several advantages over gFOBTs. Fecal immunochemical tests (FITs) specifically detect human globin so they do not require dietary restriction of meat or peroxidase-rich food and FITs typically require one to two stool samples rather than the three recommended for gFOBTs. Not surprisingly, participation rates have been reported to be significantly higher for FIT than gFOBT; 61.5 versus 49.5 % in a study by Hol et al. [28]. In addition, globin protein is digested in the stomach and proximal small bowel so FIT should be more specific for bleeding from the colon than gFOBTs. There are multiple FDA-approved FIT kits commercially available; the major technical differences among the tests are whether they can report quantitative as well as qualitative results and whether they can be performed in an individual laboratory or require central processing. The analysis of reported data with FITs is complicated by the fact that the level of sensitivity can be adjusted and the number of tests recommended is not uniform; the performance characteristics of the tests vary substantially by adjusting either or both of these parameters. FIT is typically more expensive than gFOBT but, for a single test, both are substantially less expensive than the imaging modalities described below.
Performance Characteristics
FIT is thought to have a similar sensitivity for CRCs and advanced adenomas (≥ 10 mm, presence of high-grade dysplasia or villous features) as Hemoccult SENSA and both have improved sensitivity over other gFOBTs like Hemoccult II. Allison et al. [18] reported that FIT sensitivity was higher than Hemoccult SENSA for distal CRCs (81.9 vs. 64.3 %) but lower for advanced adenomas (29.4 vs. 41.3 %). Hundt et al. [29] found great variability in the performance of six FIT kits for detection of adenomas; the two best performing tests (immoCARE-C (CARE diagnostica, Voerde, Germany) and FOB advanced (ulti med, Ahrensburg, Germany)) had sensitivities for the detection of advanced adenomas of 25 and 27 % with specificities of 97 and 93 %, respectively.
Efficacy
There are no long-term data regarding the impact of screening with FIT on CRC mortality or incidence; however, there are several trials underway with results expected in the 2020s. Results from the initial round of screening of one of the trials comparing colonoscopy with FIT (hemoglobin threshold of 75 ng/mL) showed higher compliance with the FIT arm, higher adenoma detection in the colonoscopy arm, and, after one round of FIT testing, no difference in CRC detection rates [30].
Fecal DNA Testing
Fecal DNA testing is a new and evolving stool-based screening test based on the observation that colonic neoplasms have altered DNA compared to normal cells, that colonic neoplasms shed cells into the stool, and that their DNA can be detected in stool. Fecal DNA testing has the theoretical advantage of identifying a marker thought to be in the causal pathway to CRC (mutations or mutation-like events) rather than the less specific finding of blood in the stool. Typically, an entire bowel movement is collected and shipped to a laboratory for the fecal DNA tests.
Performance Characteristics
Fecal DNA testing is a very active area of ongoing research and there are numerous studies reporting high sensitivity and specificity of various stool DNA tests in selected patient populations. In two studies using colonoscopy as a standard, a fecal DNA test (PreGen Plus®; no longer commercially available) had a sensitivity of 25–51 % for CRC and 20–41 % for clinically significant neoplasia (CRC plus advanced adenomas) with specificities of 94–96 % [31, 32]. A combination stool DNA/FIT assay (Cologuard®) was recently reported to have a 92 and 42 % sensitivity for CRC and advanced adenomas, respectively, with a specificity of 86 % [33]. This test has been submitted to the FDA for premarketing approval.
Efficacy
There are no long-term data available upon which to draw conclusions regarding the efficacy of fecal DNA testing on CRC mortality or incidence.
Blood Tests
A reliable blood test for colon cancer screening would have substantial advantages over stool collections. A large prospective study of methylated septin 9 in patients scheduled for screening colonoscopy demonstrated that a CRC marker can be detected in blood; the assay had a 48 % sensitivity for CRC [34]. A septin 9 CRC screening assay (ColoVantage®) has been submitted to the FDA for premarketing approval.
Structural Tests
Colonic imaging tests used for screening include radiologic (barium enema and CT colonography) as well as endoscopic (flexible sigmoidoscopy (FS) and colonoscopy) tests. Although barium enema is still supported as a screening modality in the multi-society guidelines [35], there are no studies evaluating its effectiveness in CRC screening and it is rarely used for screening.
Computerized Tomography Colonography
Computerized tomography colonography (CTC) emerged as a CRC screening tool in the mid-1990s, and the technology has rapidly evolved since. CTC is an attractive screening approach in that, like colonoscopy, it visualizes polyps as well as cancer throughout the colon but it does not require sedation , it takes less time, and is associated with a lower complication rate than colonoscopy . Current protocols require patients to undergo a standard bowel preparation and the colon is inflated using a rectal catheter prior to imaging, which can cause discomfort.
Performance Characteristics
Defining the sensitivity and specificity for CTC is more complicated than for any of the other screening modalities since the current radiologic practice is to not report polyps less than 5 mm in size. The reported sensitivity for polyps sized 6–9 mm has ranged from 23 to 86 % and from 52 to 92 % for polyps > 10 mm and 75–100 % for CRC [36–39]. This wide variability has been attributed to differences in technology and operator experience and training.
There is a concern that operator dependence could be even a bigger issue in the general community than that reported in the controlled trials. The trials reporting the best CTC performance [36, 39] went to great lengths to ensure that their study radiologists were highly trained and experienced with CTC. Thus, these study results may not be generalizable to the community.
Despite these concerns, the best CTC studies reported sensitivities for cancer and for polyps larger than 10 mm of 94 and 90 %, respectively, with specificites for polyps > 10 mm of 86–95 % [36–38].
Barriers to the widespread use of CTC screening include residual angst about the ability of CRC to detect diminutive and flat polyps. Even though only a small percentage of polyps less than 5 mm have advanced histology (only 1 of 966 diminutive polyps found in Pickhardt’s trial had villous features), it is unclear if leaving these polyps undetected and unremoved is acceptable to patients and their providers. There are little data about the performance of CTC for the detection of flat lesions in the colon which are increasingly reported as having a substantial cancer risk [40]. Small flat lesions are also missed frequently by endoscopy, however, and the overall sensitivity of CTC and colonoscopy for polyps > 6 mm is similar [41].
CTC is less expensive than colonoscopy but there are conflicting data regarding the cost-effectiveness of CTC compared with colonoscopy [36, 42–45]. Most of these modeling studies assumed that patients would only be referred for colonoscopy if polyps greater than 10 mm were found. In practice, Shah et al. [46] found that both patients and physicians preferred to follow even small polyps with colonoscopic examination. If all detected polyps led to colonoscopy, the cost of primary CTC screening would increase substantially.
Efficacy
There are no long-term data available to assess CTC screening on CRC mortality.
Flexible Sigmoidoscopy
FS is generally performed with a 60-cm sigmoidoscope, which typically allows visualization to the descending colon or splenic flexure (less than half of the colonic length). The bowel preparation for FS is usually enemas alone; although simpler, the preparation may not be as good as with the more extensive preparations used for CTC or colonoscopy. FS typically does not require sedation and can be performed by nonphysicians (nurses, mid-levels), but it causes more patient discomfort than sedated procedures.
Performance Characteristics
The sensitivity of FS for advanced adenomas and CRC of the entire colon is approximately 60–70 % (when compared to colonoscopy as gold standard) if colonoscopy is recommended for any adenoma detected in the distal colon [47]. Provided that the bowel preparation is good, the sensitivity and specificity for detecting lesions in the distal bowel is thought to be equivalent to colonoscopy.
Efficacy
Although earlier studies had been conflicting, three recent large controlled trials from the UK [48], Italy [49], and the USA [50] (Table 2.3) reported decreases in both incidence (18–23 %) and mortality (22–31 %) in patients randomized to FS. The benefit of FS was due to a decrease in left-sided CRCs with no significant effect on right-sided CRCs. None of the FS trials has found a statistically significant reduction in overall mortality.
Table 2.3
Summary of randomized controlled trials for flexible sigmoidoscopy: CRC incidence, mortality, and all-cause mortality (Adapted from Ref. [108]. With permission from Springer Verlag)
Trial | Participants | Follow-up (years) | CRC incidence (RRb or number) | CRC mortality (RRb) | All-cause mortality (RR or number) |
|---|---|---|---|---|---|
Telemark [113] Norway | 799 | 13 | 0.2 (95 % CI, 0.03–0.95)a | No patients died of CRC in either arm | 1.57 (95 % CI, 1.03–2.4)a |
NorCCaPc [114] Norway | 55,736 | 7 | 134.5 versus 131.9/100,000 person yr | 0.73 (95 % CI, 0.47–1.13) ITTd 0.41 (95 % CI, 0.21–0.82) per protocola | 1.02 (95 % CI, 0.98–1.07) |
UK FS trial [48] | 113,195 | 11.2 | 0.77 (95 % CI, 0.70–0.84) ITTa 0.67 (95 % CI, 0.60–0.76) per protocola | 0.69 (95 % CI, 0.59–0.82) ITTa 0.57 (95 % CI, 0.45–0.72) per protocola | 0.97 (95 % CI, 0.94–1.0) |
SCOREe Trial [49] | 34,272 | 11.4 | 0.82 (95 % CI, 0.69–0.96) ITTa 0.69 (95 % CI, 0.56–0.86) per protocola | 0.78 (95 % CI, 0.56–1.08) ITT 0.62 (95 % CI, 0.40–0.96) per protocola | 660.3 verus 641.0/100,000 person yr |
PLCOf Trial [50] US | 77,445 | 11.9 | 0.79 (95 % CI 0.72–0.85)a | 0.74 (95 % CI 0.63 to 0.87)a | 0.98 (95 % CI, 0.96–1.01) |