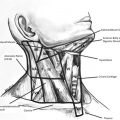
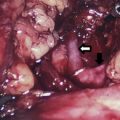
Well-differentiated thyroid cancer is increasing in incidence but the disease-specific mortality remains very low. The only effective adjuvant treatment is radioactive iodine ablation. Guidelines regarding the use and dosage of radioactive iodine depend on pathologic features of the primary and metastatic tumor that define risk. Long-term treatment includes thyroid-stimulating hormone suppression and surveillance with serum thyroglobulin and radiologic assessment for nodal recurrence.
Key points
- •
The pathology and demographics of well-differential thyroid cancer patients define specific risk groups.
- •
Adjuvant radioactive iodine is recommended for moderate- to high-risk patients after surgical resection.
- •
Administration of thyroxine to suppress TSH levels is a cornerstone of long-term therapy.
- •
Long-term follow-up is guided by serum thyroglobulin measurements and cervical ultrasound to detect recurrent disease.
Well-differentiated thyroid carcinoma (WDTC) is predominately a surgical disease with respect to the primary tumor, locoregional advanced disease, and the treatment of cervical neck recurrences. However, the multidisciplinary approach to postoperative management of thyroid cancer is central in minimizing the risk of recurrence and surveying patients long-term in a cost-effective manner to detect clinically significant disease recurrence that warrants further treatment. Because long-term survival from WDTC is good with 10-year survival of 93% for papillary thyroid cancer (PTC) inclusive of all stages it is vital to appropriately treat the patients that are at higher risk of complications from their disease burden without overtreating those patients with a low risk of thyroid cancer–related adverse outcome.
For the purpose of this article, the discussion is inclusive of the postoperative treatment of adult differentiated carcinoma processes derived from follicular epithelial cells and comprises PTC, follicular (FCC), and oncocytic follicular (Hürthle or oxyphillic cell [HCC]) subtypes.
In the past two decades, there has been a dramatic and sustained rise in incidence and detection of these well-differentiated thyroid tumors. Most of the rise in incidence is caused by the detection of PTC and likely the consequence of increased sensitivity of imaging modalities and pathologic identification of subclinical microscopic tumors. With the relative rapid increase in the diagnosis of WDTC, clinicians caring for this epidemic of thyroid cancer need guidance in providing algorithmic care from rational guidelines formed out of extensive literature review and consensus expert opinion. In our own clinical treatment of patients with thyroid cancer, postoperative care and surveillance is directed predominately by guidelines laid out by the American Thyroid Association (ATA) as initially published in 2006 and revised in 2009, with the anticipated third iteration due in 2015, and guidelines established through the National Comprehensive Cancer Network ® (NCCN ® ), with the current version published in 2015. Use of such guidelines is not to discredit the recommendations made through other vigorous reviews and the expert opinion of very relevant professional organizations involved in promoting improved WDTC care.
Assignment of risk
Most patients with WDTC who have undergone a total thyroidectomy with or without appropriate lymphadenectomy have relatively excellent prognosis for long-term survival and very low disease-specific mortality. However, there is an inherent risk of growth of occult persistent disease and this is often reflected as a locoregional cervical neck or mediastinal recurrence. This is especially true for PTC, which accounts for most cases of WDTC. As reasoned, the ultimate goal in the postoperative management of patients with WDTC is to maximize disease-free recurrence with appropriate and measured treatments that serve benefit to those patients with a real risk of adverse outcome from recurrent disease while not overtreating most patients that have minimal risk of recurrence following surgical removal of the primary tumor alone.
Specific factors have been identified that permit a more individualized estimation of recurrence rate and survival and help guide long-term oncologic adjuvant treatment and surveillance strategies. Of these, the patient’s age at the time of presentation and tumor stage are two of the most important prognostic factors. As one would expect, age is an independent variable in the long-term risk of mortality, with older patients more likely to succumb to disease burden. The inflection point of increased risk of death begins in the fourth decade and escalates for those greater than 60 years of age. Risk of tumor recurrence, however, is more bimodal as it pertains to age at diagnosis. The risk of recurrence is highest for those with age less than 20 and greater than 60. Male sex also portends more aggressive disease when compared with that of female cohorts.
As with any other cancer, tumor biology has a profound influence on the expected long-term outcome. For WDTC, this is best represented by the pathologic features of the primary tumor including tumor size, tumor histology, extrathyroidal extension (ETE), vascular invasion, and the extent of metastatic lymph node involvement. Clearly, small tumors (<1 cm) conventionally referred to as microcarcinomas would be expected to have favorable outcomes and are addressed separately. Larger tumors (>1.5 cm) are associated with a higher risk of recurrence and disease-specific mortality. Indeed, there is an incremental increase in the risk of distant metastasis with increasing tumor size.
Certain histologic variants of WDTC portend a more concerning risk of recurrence and/or disease-specific mortality. Among these are variants of PTC including tall-cell, insular, columnar, and diffuse sclerosing variants. Conversely, follicular variant PTC carries a very favorable risk profile and is especially true with evidence of clear demarcation or complete encapsulation. Conventional follicular and oncocytic follicular carcinomas are largely considered to have more aggressive tumor biology especially with larger tumors and advanced age. However, tumors with evidence of minimal invasion as defined by microscopic capsular disruption are considered to have a favorable biology and low risk of recurrence.
Ten percent of WDTC exhibit evidence on anatomic pathology of tumor extension through the outer thyroid capsule into the immediate perithyroidal soft tissue. Such extension is variable and designated as either minimal ETE (American Joint Committee on Cancer [AJCC] T3) or macroscopic and extensive (AJCC T4a). The risk of recurrence significantly correlates to the degree of ETE. For minimal ETE, risk of recurrence is 3% to 9%, whereas recurrence rates for T4a disease is 23% to 40%. Further, intrathyroidal extension observed as vascular invasion has been shown to correlate with tumor recurrence (16%–30%), high rate of distant metastasis (12%–35%), and long-term adverse outcomes.
Lymph node metastasis in the context of WDTC is common and observed in most patients (62%–81%) when detailed lymph node dissection is performed. However, it is clear from large retrospective series and observational studies that not all lymph node metastasis carry the same clinical significance because the real risk of recurrence ranges from 2% to 38%. To an extent, this holds true even in patients managed without lymph node dissection or adjuvant treatment with radioactive iodine (RAI). To more clearly synthesize the abundant and varied data for lymph node metastasis and risk of recurrence or adverse outcome, an ATA taskforce clarified the characteristics of lymph node metastasis to be considered at low risk or higher risk. Micrometastasis (<0.2 cm in largest diameter) in five or fewer nodes are classified as lower-risk disease with an estimated risk of recurrence of 5% without further treatment. More than five metastatic lymph nodes, clinically apparent N1 disease (detected with either preoperative imaging or intraoperatively), or any metastatic focus greater than 3 cm are classified as higher risk of recurrence with risk of recurrence greater than 20%. Identification of extranodal extension is also an important indication of biology and the risk of recurrence is 2% for patients with less than or equal to three lymph nodes exhibiting extranodal extension rising to 38% for those with greater than three lymph nodes demonstrating extranodal extension.
The extent of data available regarding the various risk factors for recurrence and disease-specific morbidity for a given presentation of WDTC has resulted in several different staging systems all establishing a relative prognostic score. The AJCC TNM staging system is considered the most useful for prediction of the risk of death from thyroid cancer. However, the AJCC TNM staging system does tend to fall short in its overall clinical relevance in predicting recurrence of WDTC. Other common prognostic scoring methodologies used within the literature in analyzing large prospective and retrospective clinical cohorts include AGES ( A ge, tumor G rade, E xtent, and S ize), AMES ( A ge, M etastasis, E xtent, and S ize), MACIS ( M etastasis, A ge, C ompleteness of resection, I nvasion, and S ize), EORTC (European Organization for Research and Treatment of Cancer), and NTCTCS (National Thyroid Cancer Treatment Cooperative Study) among other more institutional-specific scores (Ohio State University and Memorial Sloan Kettering Cancer Center). Such scoring systems alternatively weigh the typical prognostic variables to better define the outcome end point they were designed to measure. Overall, none of the scoring systems have shown over time to provide primacy in the ability to predict outcome whether it is recurrence or disease-specific mortality. A simplified three-tier risk staging system was proposed as part of the 2009 ATA guidelines ( Table 1 ) to more specifically address the risk of clinical recurrence and has proved to be well validated in its approach. This more relevant risk stratagem provides the basis for the key components in the postoperative care of WDTC patients as it pertains to adjuvant treatment with RAI and thyroid-stimulating hormone (TSH) suppression, in addition to establishing the tempo and intensity of long-term surveillance.
| Low Risk | Intermediate Risk | High Risk |
|---|---|---|
| Absence of local or distant metastasis Complete macroscopic tumor resection No tumor invasion of locoregional tissues or structures Absence of vascular invasion or predominate aggressive histology a No I 131 uptake outside the thyroid bed on posttreatment whole-body RAI scan | Evidence of microscopic extrathyroidal extension into perithyroidal tissue Presence of cervical lymph node metastasis Evidence of aggressive histology a or vascular invasion Evidence of I 131 uptake outside the thyroid bed on posttreatment whole-body RAI scan | Macroscopic tumor invasion of perithyroidal structures Incomplete resection of the primary tumor Distant metastasis Serum Tg levels out of proportion to I 131 uptake observed on posttreatment whole-body RAI scan |
a Insular, columnar, tall-cell, solid, and diffuse sclerosing variants.
An important consideration in long-term postoperative care of WDTC patients is to be cognizant that the initial staging as specified by the AJCC TNM stage does not change over time; however, because most patients live decades following diagnosis, the biology of the disease may evolve with time. Most WDTC persistent disease remains indolent in nature and escalation of treatment is infrequently needed. For some patients with thyroid cancer, nonetheless, the response to therapy is incomplete and/or the clinical course transforms and with that the ongoing risk of recurrence, disease progression, and risk of disease-specific death may change over time. Thus, the multidisciplinary team tasked with long-term treatment of each patient should provide an ongoing reassessment of the risk of recurrence as their clinical data emerges with the response to therapy and clinical course.
Assignment of risk
Most patients with WDTC who have undergone a total thyroidectomy with or without appropriate lymphadenectomy have relatively excellent prognosis for long-term survival and very low disease-specific mortality. However, there is an inherent risk of growth of occult persistent disease and this is often reflected as a locoregional cervical neck or mediastinal recurrence. This is especially true for PTC, which accounts for most cases of WDTC. As reasoned, the ultimate goal in the postoperative management of patients with WDTC is to maximize disease-free recurrence with appropriate and measured treatments that serve benefit to those patients with a real risk of adverse outcome from recurrent disease while not overtreating most patients that have minimal risk of recurrence following surgical removal of the primary tumor alone.
Specific factors have been identified that permit a more individualized estimation of recurrence rate and survival and help guide long-term oncologic adjuvant treatment and surveillance strategies. Of these, the patient’s age at the time of presentation and tumor stage are two of the most important prognostic factors. As one would expect, age is an independent variable in the long-term risk of mortality, with older patients more likely to succumb to disease burden. The inflection point of increased risk of death begins in the fourth decade and escalates for those greater than 60 years of age. Risk of tumor recurrence, however, is more bimodal as it pertains to age at diagnosis. The risk of recurrence is highest for those with age less than 20 and greater than 60. Male sex also portends more aggressive disease when compared with that of female cohorts.
As with any other cancer, tumor biology has a profound influence on the expected long-term outcome. For WDTC, this is best represented by the pathologic features of the primary tumor including tumor size, tumor histology, extrathyroidal extension (ETE), vascular invasion, and the extent of metastatic lymph node involvement. Clearly, small tumors (<1 cm) conventionally referred to as microcarcinomas would be expected to have favorable outcomes and are addressed separately. Larger tumors (>1.5 cm) are associated with a higher risk of recurrence and disease-specific mortality. Indeed, there is an incremental increase in the risk of distant metastasis with increasing tumor size.
Certain histologic variants of WDTC portend a more concerning risk of recurrence and/or disease-specific mortality. Among these are variants of PTC including tall-cell, insular, columnar, and diffuse sclerosing variants. Conversely, follicular variant PTC carries a very favorable risk profile and is especially true with evidence of clear demarcation or complete encapsulation. Conventional follicular and oncocytic follicular carcinomas are largely considered to have more aggressive tumor biology especially with larger tumors and advanced age. However, tumors with evidence of minimal invasion as defined by microscopic capsular disruption are considered to have a favorable biology and low risk of recurrence.
Ten percent of WDTC exhibit evidence on anatomic pathology of tumor extension through the outer thyroid capsule into the immediate perithyroidal soft tissue. Such extension is variable and designated as either minimal ETE (American Joint Committee on Cancer [AJCC] T3) or macroscopic and extensive (AJCC T4a). The risk of recurrence significantly correlates to the degree of ETE. For minimal ETE, risk of recurrence is 3% to 9%, whereas recurrence rates for T4a disease is 23% to 40%. Further, intrathyroidal extension observed as vascular invasion has been shown to correlate with tumor recurrence (16%–30%), high rate of distant metastasis (12%–35%), and long-term adverse outcomes.
Lymph node metastasis in the context of WDTC is common and observed in most patients (62%–81%) when detailed lymph node dissection is performed. However, it is clear from large retrospective series and observational studies that not all lymph node metastasis carry the same clinical significance because the real risk of recurrence ranges from 2% to 38%. To an extent, this holds true even in patients managed without lymph node dissection or adjuvant treatment with radioactive iodine (RAI). To more clearly synthesize the abundant and varied data for lymph node metastasis and risk of recurrence or adverse outcome, an ATA taskforce clarified the characteristics of lymph node metastasis to be considered at low risk or higher risk. Micrometastasis (<0.2 cm in largest diameter) in five or fewer nodes are classified as lower-risk disease with an estimated risk of recurrence of 5% without further treatment. More than five metastatic lymph nodes, clinically apparent N1 disease (detected with either preoperative imaging or intraoperatively), or any metastatic focus greater than 3 cm are classified as higher risk of recurrence with risk of recurrence greater than 20%. Identification of extranodal extension is also an important indication of biology and the risk of recurrence is 2% for patients with less than or equal to three lymph nodes exhibiting extranodal extension rising to 38% for those with greater than three lymph nodes demonstrating extranodal extension.
The extent of data available regarding the various risk factors for recurrence and disease-specific morbidity for a given presentation of WDTC has resulted in several different staging systems all establishing a relative prognostic score. The AJCC TNM staging system is considered the most useful for prediction of the risk of death from thyroid cancer. However, the AJCC TNM staging system does tend to fall short in its overall clinical relevance in predicting recurrence of WDTC. Other common prognostic scoring methodologies used within the literature in analyzing large prospective and retrospective clinical cohorts include AGES ( A ge, tumor G rade, E xtent, and S ize), AMES ( A ge, M etastasis, E xtent, and S ize), MACIS ( M etastasis, A ge, C ompleteness of resection, I nvasion, and S ize), EORTC (European Organization for Research and Treatment of Cancer), and NTCTCS (National Thyroid Cancer Treatment Cooperative Study) among other more institutional-specific scores (Ohio State University and Memorial Sloan Kettering Cancer Center). Such scoring systems alternatively weigh the typical prognostic variables to better define the outcome end point they were designed to measure. Overall, none of the scoring systems have shown over time to provide primacy in the ability to predict outcome whether it is recurrence or disease-specific mortality. A simplified three-tier risk staging system was proposed as part of the 2009 ATA guidelines ( Table 1 ) to more specifically address the risk of clinical recurrence and has proved to be well validated in its approach. This more relevant risk stratagem provides the basis for the key components in the postoperative care of WDTC patients as it pertains to adjuvant treatment with RAI and thyroid-stimulating hormone (TSH) suppression, in addition to establishing the tempo and intensity of long-term surveillance.
| Low Risk | Intermediate Risk | High Risk |
|---|---|---|
| Absence of local or distant metastasis Complete macroscopic tumor resection No tumor invasion of locoregional tissues or structures Absence of vascular invasion or predominate aggressive histology a No I 131 uptake outside the thyroid bed on posttreatment whole-body RAI scan | Evidence of microscopic extrathyroidal extension into perithyroidal tissue Presence of cervical lymph node metastasis Evidence of aggressive histology a or vascular invasion Evidence of I 131 uptake outside the thyroid bed on posttreatment whole-body RAI scan | Macroscopic tumor invasion of perithyroidal structures Incomplete resection of the primary tumor Distant metastasis Serum Tg levels out of proportion to I 131 uptake observed on posttreatment whole-body RAI scan |
a Insular, columnar, tall-cell, solid, and diffuse sclerosing variants.
An important consideration in long-term postoperative care of WDTC patients is to be cognizant that the initial staging as specified by the AJCC TNM stage does not change over time; however, because most patients live decades following diagnosis, the biology of the disease may evolve with time. Most WDTC persistent disease remains indolent in nature and escalation of treatment is infrequently needed. For some patients with thyroid cancer, nonetheless, the response to therapy is incomplete and/or the clinical course transforms and with that the ongoing risk of recurrence, disease progression, and risk of disease-specific death may change over time. Thus, the multidisciplinary team tasked with long-term treatment of each patient should provide an ongoing reassessment of the risk of recurrence as their clinical data emerges with the response to therapy and clinical course.
The incidental papillary thyroid microcarcinoma
The detection of small PTC lesions (<1 cm), termed microcarcinomas (PTMC), has been steadily increasing over the last four decades and accounts for a large portion of the steady rise observed in overall number of PTC over that same timeframe. These are typically found incidental to the original indication for thyroid surgery and are very-low-risk lesions with essentially zero risk of disease-specific mortality and less than 1% risk of distant metastasis. The risk of locoregional recurrence is 2.4% despite studies reporting 60% of PTMC are associated with lymph node metastasis. Patients with PTMC have an excellent overall prognosis and most pertinent studies demonstrate no significant improvement in recurrence rate or increased survival after total thyroidectomy versus thyroid lobectomy with or without RAI treatment in the context of PTMC. Accordingly, the ATA and NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ® ) do not advocate the use of RAI ablation or completion thyroidectomy for these tumors because of the low risk of recurrence and metastatic potential. If PTMC is discovered following diagnostic lobectomy whether unifocal or multifocal, periodic surveillance of the contralateral lobe with high-resolution ultrasound (US) is adequate for postsurgical management.
Completion thyroidectomy
Not all patients have the amenity of a preoperative diagnosis of WDTC and anywhere from 5% to 75% of patients with nondiagnostic, atypia of undetermined significance, follicular lesion, or suspicious for malignancy cytology (Bethesda System for Reporting Thyroid Cytopathology I, III, IV, and V) on fine-needle aspiration (FNA) biopsy have a clinically significant malignancy diagnosed on anatomic pathology following the initial surgical resection. Many of these patients receive a diagnostic lobectomy because of the uncertainty of the indeterminate cytology reporting categories. For these patients, the first step in postoperative care involves the decision to proceed with completion thyroidectomy. This often requires a multidisciplinary approach and clear communication between the endocrine surgeon, endocrinologist, and nuclear medicine specialist involved in posttreatment plans.
Total thyroidectomy for patients with primary tumors greater than or equal to 1 cm yields lower recurrence rates and higher survival rates. As such, the treatment of choice is total thyroidectomy if the tumor is biopsy proved preoperatively. Current guidelines prescribe that if a total thyroidectomy was warranted with a preoperative diagnosis of WDTC, then completion thyroidectomy should be offered. This is especially true for those patients that are to be recommended RAI treatment or those where active surveillance is more of a concern. Conversely, patients with low-risk purely intrathyroidal PTC (favorable histology, such as follicular variant <4 cm) and without evidence of ETE or only minimally invasive FCC less than 2 cm may be considered to have definitive therapy with thyroid lobectomy alone.
Treatment or attempt at ablation of the remaining contralateral lobe of the thyroid with RAI is not recommended under circumstances where it can be removed surgically. Circumstances that can present a challenge with respect to completion thyroidectomy are cases where the recurrent laryngeal nerve is injured at the initial resection and the patient is left with a vocal fold motion deficit. This also highlights the vital importance of laryngoscopy in the postoperative setting to document any deficit and proceed with treatment if needed.
Radioactive iodine treatment
In the context of surgical oncology and adjuvant oncology treatment, there are few treatments that are more “targeted” than that of RAI. A hallmark of follicular-derived thyroid epithelial cells is their ability to import via the sodium-iodine symporter and retain iodine in the process of organification. Such activity provides for a reasonable cellular retention time and potentiates radiopharmacologic imaging and treatment. Postoperative treatment with radioactive I 131 is primarily applied for three reasons. First, RAI ablates or eliminates any remaining normal thyroid tissue and facilitates the specificity of thyroglobulin (Tg) as a tumor marker in long-term surveillance. This is of more significance in the low-risk and some intermediate-risk group patients that otherwise may never have disease recurrence but could observe an increase in Tg over time because of growth of any normal thyroid remnant tissue from incomplete thyroidectomy. Second, RAI serves as an adjuvant treatment of intermediate-risk patients to destroy remaining occult small foci of WDTC and potentiating a decrease in long-term risk of recurrent disease. Finally, RAI may be administered in a true therapeutic fashion for those high-risk patients with macroscopic residual disease or distant metastatic disease.
The 2009 ATA guidelines and current version of NCCN Guidelines for the treatment of WDTC patients with RAI are reasonably congruent ( Table 2 ). In general, both sets of guidelines define a set of patients at low risk of recurrence for whom there is little documented benefit in administering either an ablative or adjuvant dose of RAI. Certainly for those patients at high risk of recurrence there is relative uniformity in recommending an empiric therapeutic dose of RAI. For all other WDTC patients in between as either low risk with tumors 1 to 4 cm or intermediate risk with only a low-volume of nodal metastasis, the use becomes more selective and inherently subjective. Furthermore, for those patients in the intermediate-risk group or those with low risk of recurrence but tumors greater than 4 cm the multidisciplinary team must determine the appropriate empiric dose range to treat, whether that is more consistent with an ablative dose (30–100 mCi) or an adjuvant dose (100–200 mCi).
| Not Recommended | Selective Use | Recommended | Recommended | |
|---|---|---|---|---|
| Ablative (30–100 mCi) or adjuvant dosing (100–200 mCi) | Ablative (30–100 mCi) or adjuvant dosing (100–200 mCi) | Therapeutic dosing 100–200 mCi | ||
| ATA c | Most low risk | Low risk with tumor 1–4 cm a Intermediate risk: age <45 nodal metastasis only a Intermediate risk: age >45 nodal metastasis only (AJCC T1-2 N1a) a Intermediate risk: any age with minimal ETE only (AJCC T3 with tumor <4 cm) a | Low risk with tumor >4 cm a Most intermediate risk a | High risk |
| [NCCN] d | PTC: unifocal or multifocal classic variant PTC with all primary tumors <1 cm without ETE, absence of anti-Tg antibodies and unstimulated Tg <1 ng/mL FCC/HCC: primary tumor <2 cm without ETE or vascular invasion, absence of nodal or distant metastasis and absence of anti-Tg antibodies | PTC: tumor 1–4 cm or high-risk histology or lymphovascular invasion or lymph node metastasis or macroscopic multifocal (>1 cm) or positive anti-Tg antibodies or unstimulated Tg 1–5 ng/mL b FCC/HCC: primary tumor 2–4 cm or minor vascular invasion or lymph node metastasis or positive anti-Tg antibodies or unstimulated Tg 1–5 ng/mL b | PTC: primary tumor >4 cm or gross extrathyroidal extension or unstimulated Tg >5 ng/mL b FCC/HCC: primary tumor >4 cm or gross extrathyroidal extension or extensive vascular invasion or unstimulated Tg >5 ng/mL b | PTC, FCC, and HCC: known or suspected distant metastasis or incomplete resection or primary tumor |
a Suspected or known residual disease based on clinical judgment or demonstrated on pretherapy WBS should prompt treatment with 100 to 200 mCi. In the absence of known or suspected residual disease based on clinical judgment or pretherapy WBS, dosing of 30 to 100 mCi should be used.
b If pretherapy WBS shows no thyroid bed uptake and Tg <1 ng/mL then follow without ablation. With thyroid bed uptake only proceed with remnant ablation dose of 30 mCi. For patients with proved metastatic disease proceed with empiric dosing 100–200 mCi.
c Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. PMID: 19860577 .
d Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ® ) for Thyroid Carcinoma V.2.2015 © National Comprehensive Cancer Network, Inc 2015. All rights reserved. Accessed September 14, 2015. To view the most recent and complete version of the guideline, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK ® , NCCN ® , NCCN GUIDELINES ® , and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree