Sleep disorders
Valerian (Valeriana officinalis), lemon balm (Melissa officinalis)
Nervous agitation
Purple passion flower (Passiflora incarnata)
Depressed mood
Common St John’s wort (Hypericum perforatum)
Urinary tract infections
Cranberry (Vaccinium marcrocarpon)
Irritable bladder
Pumpkin (Cucurbita pepo)
Hepatic disease
Mary Thistle (Silybum marianum)
Dyspepsia, bile flow
Artichoke (Cynara scolymus)
Pain, fever
White willow bark (Salix alba)
Inflammation, rheumatism
Devil’s claw (Harpagophytum procumbens)
Blunt injuries
Common comfrey (Symphytum officinale)
Premenstrual syndrome
Chaste tree (Vitex agnus-castus)
Menopausal symptoms
Black cohosh (Cimicifuga racemosa), soybean (Glycine max), red clover (Trifolium pratense)
21.1 ‘Phytoestrogens’? A Misleading Term
The idea that plants could have effects ‘like oestrogen’ ultimately dates back to observations anomalies in the menstrual cycles of hop pickers. The common hop (Humulus lupulus) contains the ingredient 8-Prenylnaringenin, a flavonoid of which very large quantities can in fact activate the oestrogen alpha receptor, triggering hormonal effects in the process. These effects may be difficult to achieve with the usual amounts of hop extracts (those found in plant-based sedatives, for instance).
In 1946, when fertility problems were observed in sheep in Australia, based on experience with hops, the effect was quickly attributed to a plant that is used to treat the symptoms of menopause: the red clover (Trifolium pratense) found growing in the meadows where the sheep were grazing [1]. The discrepancy that European livestock also graze on red clover, yet that the plant fails to trigger any fertility problems here, was never discussed and should have prompted massive doubts about the attribution of blame to red clover. Red clover contains isoflavones, isoflavones help alleviate menopausal symptoms; ergo and isoflavones have contraceptive endocrine effects. This is how a plant that is otherwise in good standing can slide into disrepute. In point of fact, the effects of isoflavones are manifested not via the oestrogen alpha receptor; instead, they activate the oestrogen beta receptor.
In contrast to soya and red clover, other plant extracts used in menopause exhibit other mechanisms: black cohosh (Cimicifuga racemosa) does not work through oestrogen receptors at all; rhapontic rhubarb (Rheum rhaponticum), on the other hand, with the ingredient rhaponticin, contains stilbestrols, which in turn activate the oestrogen alpha receptor. The term ‘phytoestrogen’ would be more fitting in the case of rhubarb but not for the isoflavones found in red clover or soya. A more apt designation here would be that of ‘selective oestrogen receptor modulators’ (phytoSERMs).
21.2 The Oestrogen Beta Receptor Protects Against Excessive Hormonal Effects
Classic oestrogen effects such as the proliferation of breast and uterine tissue are controlled via the oestrogen alpha receptor. The oestrogen beta receptor is activated by oestrogen as well. The effects that this produces protect against excessive activation of the alpha receptor, for instance, during cycle phases involving high oestrogen levels. The oestrogen beta receptor was only discovered in 1996 [2], and soon thereafter, it was found that isoflavones can activate this protective receptor type [3]. The oestrogen beta receptor is expressed primarily in hormone-sensitive tissues, such as the breast and the uterus, and also in the bones and in the cardiovascular system.
21.3 The Significance of 3β Adiol in Menopause
Estradiol is not the only physiological binding partners on the ER-β. The research findings of recent years point to a complex pattern of activation and modulation of the oestrogen receptors not only by oestrogen itself but also by a metabolite of androgens: 5α-androstan-3β, 17β-diol, also known as ‘3β adiol’. This metabolite no longer has any androgenic effects, but it does not trigger any oestrogenic effects on ER-α or -3β adiol [4], a specific ER-β agonist with a contribution to hormonal balance in the female organism.
In fact, 3β adiol is formed in the female organism even before the emergence of significant quantities of oestrogen in puberty. During the fertile years, blood levels of 3β adiol parallel those of oestrogen [5, 6]. This parallel development could contribute to an understanding of why, despite the proliferation-promoting effects of oestrogen at ER-α, the regular occurrence of very high levels of oestrogen in puberty and during the menstrual cycle and pregnancy does not lead to an increased incidence of cancer. During menopause, on the other hand, levels of both hormones, oestrogen and 3β adiol, are reduced [7, 8].
21.3.1 Protective Effect of Isoflavones
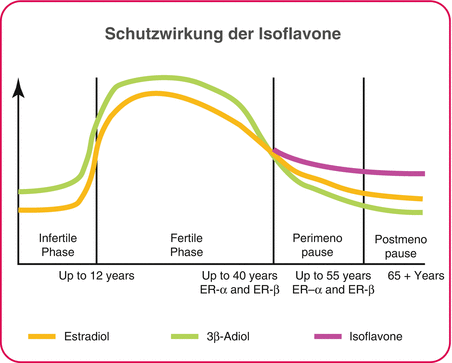
Thus, the onset of menopausal symptoms correlates not only with reduced levels of oestrogen but also with reduced 3β adiol levels [9] – and hence with a reduced activation of ER-β (Fig. 21.1).
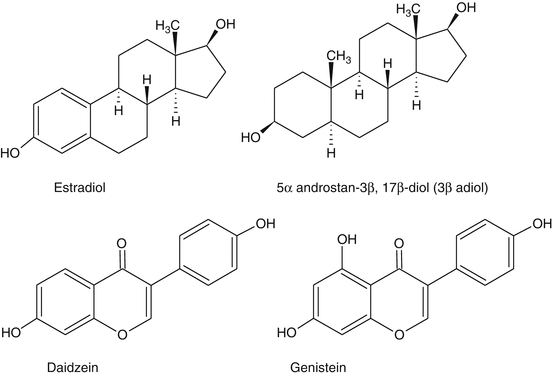

Fig. 21.1
Structures of estradiol and 3β adiol and of the isoflavones genistein and daidzein
Activation of ER-beta by high oestrogen levels, by 3β adiol, as well as by isoflavones does the following:
Triggers protective effects against excessive proliferation of breast or uterine tissue
Counteracts increased bone resorption due to oestrogen
Produces protective effects in the cardiovascular system
Reduces vasomotor complaints during menopause
The connection between soya and a reduced incidence of menopausal symptoms and also between osteoporosis and hormone-related types of cancer such as breast cancer and uterine cancer has long since been identified in epidemiological investigations, particularly in studies from Asian countries. Early on, this effect was attributed to the group of isoflavones; the typical daily intake as part of a traditional Asian diet is 30–100 mg (calculated as genistein), while a Western diet typically reaches amounts of no more than 1–2 mg per day. The quantities consumed in Asia are sufficient to activate the oestrogen beta receptor [10]; a Western diet is not expected to have this result.
21.4 Reduction of Menopausal Symptoms Demonstrated Beyond a Doubt
The reduction of menopause-related hot flashes through isoflavones has been clearly documented. In a currently published meta-analysis, ten double-blind studies were subjected to a more precise statistical review, the result of which was that there can be no doubt of the superiority of isoflavones to placebo [11]. This was quantified in further current reviews of 16 or 17 double-blind studies; in terms of the reported frequency and severity of hot flashes, isoflavones were 25–26 % more effective than placebo, and they achieved 57 % of the potency of oestrogen [12, 13]. With this in mind, the Austrian Menopause Association [Menopausegesellschaft] has thus recommended that isoflavones be used first in the treatment of menopausal symptoms before hormone replacement therapy is considered [14].
21.4.1 Isoflavones: Proven Safety in Controlled Studies
Hormone-based treatment was called into question as a result of the WHI study after it was found that the administration of hormones increased the risk of stroke and breast cancer [15]. This was subsequently corrected by identifying the increased risk as the product not of the oestrogen component but of the combination with a synthetic progestogen component [16] – and yet, for reasons that defy logic, the WHI study was used as an occasion to attribute the same kind of risk to the isoflavones derived from soya and red clover as well. Since that time, the hormonal safety of isoflavones has formed the subject of a multiplicity of studies: not only was the absence of tumour-promoting effects documented, but also isoflavones were shown to have preventive effects. The experimental investigations on animals have even taken on a curative purpose; a recent study most impressively demonstrated the decline in the size of tumours in animal breast tissue concomitant to increasing quantities of genistein [17]. This is consistent with the current development of genistein derivatives for use as angiogenesis inhibitors in cancer therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree