Cryosurgical Ablation of Bone Tumors
Jacob Bickels
Yair Gortzak
Yehuda Kollender
Martin M. Malawer
BACKGROUND
Cryoablation is the therapeutic application of cold in situ to induce tissue necrosis with curative intent. Cryoablation of bone tumors by direct pour of liquid nitrogen is an effective adjuvant to curettage in the management of a large variety of bone tumors including benign aggressive, metastatic, and primary malignant lesions. It is an intralesional procedure, which enables the avoidance of major resection and associated loss of function.
It is a very powerful technique that weakens the bone surrounding the tumor cavity and may, when not used judiciously, cause additional soft tissue injuries. Awareness of these potential complications has led to refinement of surgical practices to include soft tissue protection, stable reconstruction, and the use of perioperative antibiotics and enhancement of rehabilitation protocols for gradual weight bearing. Those guidelines resulted in a gratifying low rate of complications and rendered this treatment a safe and reliable modality.
It may be expected that cryoablation will no longer be the exclusive practice of a relatively small group of surgeons and that it will eventually enjoy greater popularity in the not too distant future.
Historical Aspects and Physiologic Background
Although it had been used in the 1850s for the management of locally advanced carcinoma of the cervix, the applicability of cryoablation in the management of bone tumors was not assessed until more than a century later, in the classic 1966 animal study by Gage et al13 in which the femora of living mongrel dogs were frozen by perfusing liquid nitrogen through encircling latex coils. Liquid nitrogen, which has a boiling temperature of -196 °C, allowed rapid freezing of a 2-cm rim of bone around these coils. Using histopathologic studies and plain radiographs, the authors documented the occurrence of tissue necrosis and bone resorption that was associated with mechanical weakening and spontaneous fractures.13 These changes, however, were followed by new bone formation that developed slowly, starting from the vital bone at the periphery: It was first observed at 2 months and reached its peak at 6 months after freezing.13
Although only normal bone was investigated in their experiment, Gage et al13 speculated that cold allows for nonspecific cell destruction and may induce tumor kill as well. They further suggested the use of intralesional cryoablation in lieu of tumor resection or amputation. The use of this technique in the management of human bone tumors was first reported in 1969.33 Following curettage of a metastatic bone lesion, those authors poured liquid nitrogen into the tumor cavity with the intent of inducing tumor necrosis and avoiding the need for extensive resection and reported having achieved both goals.
Further studies confirmed and refined the initial findings of Gage et al13 and showed that temperatures between -21 °C and -60 °C are needed to obtain cell necrosis, whereas temperatures below -60 °C exerted no further lethality.18,28,33
It also emerged that a number of mechanisms are responsible for the tissue necrosis induced by cryoablation.12,15,22,26,28,41,42 These mechanisms can be grouped into two categories: immediate and delayed.
Four mechanisms are involved in the immediate cytotoxicity produced by cryoablation: (1) formation of ice crystals and membrane disruption, (2) thermal shock, (3) dehydration and toxic effects of electrolyte changes, and (4) denaturation of cellular proteins. The formation of intracellular ice crystals is considered as being the main mechanism of immediate cellular necrosis.
During cryoablation, ice crystals first occur in the extracellular spaces. The withdrawal of water from the system into these crystals creates a hyperosmotic extracellular environment, which, in turn, draws water from the cells. As the process continues, these crystals grow, the cells shrink and dehydrate, electrolyte concentration is increased, and membranes and cell constituents are damaged.12,17,41 Rapid freezing, such as that achieved by direct pour of liquid nitrogen, does not allow sufficient time for the withdrawal of water from the cells and so intracellular ice crystals are formed simultaneously. Conversely, a slow thaw will cause intracellular recrystallization of the already formed crystals and membrane disruption, whereas a rapid thaw will not.2,7,41,48 Repeated freeze-thaw cycles will also increase the extent of tissue necrosis because of the improved cold conductivity following the first cycle.11,12,15 Therefore, repeated cycles of rapid freezing and spontaneous thaw will achieve the maximal effect of cell necrosis.
Histologically, the most dramatic effect of cryoablation is on the appearance of the bone marrow: a rim of 1 to 2 cm of extensive necrosis with minimal inflammatory response appears, following direct pour of liquid nitrogen.13,29,39,41 This is followed by liquefaction and progressive fibrosis. Large, thickened, and thrombosed vessels are occasionally seen as well.
INDICATIONS
Histologic Diagnoses
Benign aggressive bone tumors
Giant cell tumor
Aneurysmal bone cyst
Simple bone cyst
Fibrous dysplasia
Enchondroma
Chondroblastoma
Eosinophilic granuloma
Osteoblastoma
Chondromyxoid fibroma
Low-grade sarcomas of bone
Low-grade chondrosarcoma
Metastatic tumors
Morphologic Criteria
Cryoablation is appropriate for periarticular and sacral lesions in which the circumferential rim of the cortex that remained after tumor removal could hold liquid material and was sufficient for ensuring a mechanically stable reconstruction.
SURGICAL MANAGEMENT
Tumor exposure
Thorough curettage
High-speed burr drilling of the tumor cavity
Cryoablation
Mechanical reconstruction
Cryoablation using direct pour of liquid nitrogen has several technical drawbacks.
First, after it has been poured, there is no control of the overall freezing time or of the temperature at different sites within the tumor cavity.
Second, it is a gravity-dependent procedure, that is, the poured liquid cannot reach corners of the tumor cavity that are positioned above the fluid level.
To solve these problems, closed cryoablation using argon gas was developed and became available in the late 1990s.4
Both techniques are considered in the following text.
TECHNIQUES
▪ Direct Pour of Liquid Nitrogen
Exposure
When technically possible, a pneumatic tourniquet is used during the procedure to decrease local bleeding and prevent blood from acting as a heat sink and posing as a thermal barrier to cryoablation.
A large cortical window the size of the longest longitudinal dimension of the tumor is made after exposure of the involved bone. It has to be elliptical with its axis parallel to the long axis of bone to reduce the stress rising effect (TECH FIG 1).
Curettage and Burr Drilling
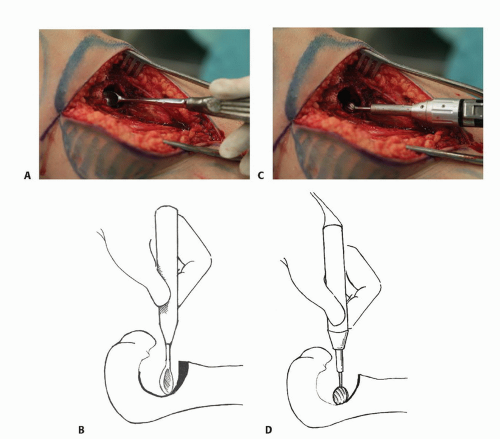
All gross tumor material is removed with hand curettes (TECH FIG 2A,B).
This is followed by high-speed burr drilling of all remaining macroscopic disease and the walls of the tumor cavity (TECH FIG 2C,D).
Bony perforations are identified and sealed with Gelfoam (Upjohn, Kalamazoo, MI) before introduction of the liquid nitrogen. Neurovascular bundle and fasciocutaneous flaps are protected by mobilization and by shielding (with surgical pads) from direct contact with the liquid nitrogen, after which cryoablation is performed.
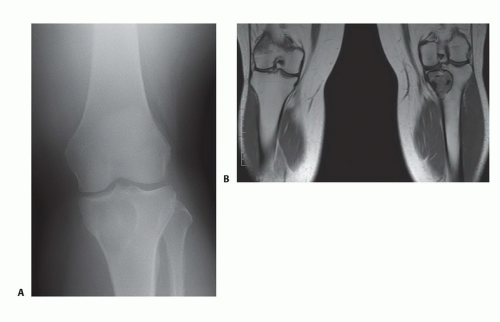 TECH FIG 1 • Plain radiograph (A) and magnetic resonance imaging (MRI) showing (B) giant cell tumor of the proximal tibia. (continued) |
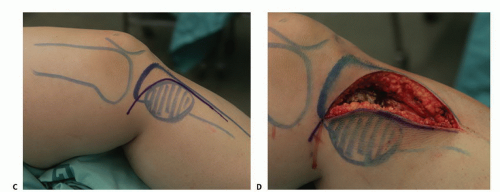 TECH FIG 1 • (continued) C. A large incision is planned to allow wide exposure of the tumor cavity. D. Fasciocutaneous flaps are mobilized and the cortical bone overlying the tumor cavity is exposed. |
Cryoablation
The traditional technique of cryoablation entails direct pour of liquid nitrogen through a stainless steel funnel into the tumor cavity, taking care to fill the entire cavity (TECH FIG 3A,B). Thermocouples are used to monitor the freeze within the cavity, cavity wall, adjacent soft tissues, and an area 1 to 2 mm from the periphery of the cavity. The surrounding soft tissues are continuously irrigated with warm saline solution to decrease the possibility of thermal injury.
Freezing (boiling of liquid nitrogen) lasts 1 to 2 minutes and is proportional to the volume of poured liquid nitrogen. It is followed by spontaneous thaw that occurs over 3 to 5 minutes. The cycle is considered complete once the temperature of the cavity rises more than 0°C. The cavity is irrigated with saline after two freeze-thaw cycles have been carried out (TECH FIG 3C-E). At this point, the process of reconstructing the tumor cavity begins.
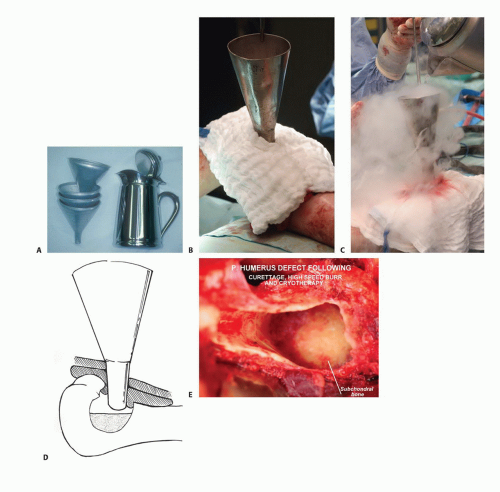
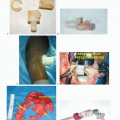
TECH FIG 3 • The traditional technique of cryoablation using direct pour of liquid nitrogen. A. Stainless steel can and funnels. B. The surrounding soft tissues are protected with surgical pads. C. Direct pour of liquid nitrogen into the tumor cavity, which is continuously irrigated with warm saline throughout the freezing and thawing processes (˜5 minutes altogether). D. Illustration showing direct pour of liquid nitrogen and protection of the surrounding soft tissues. E. Intraoperative photograph following curettage, high-speed burr, and cryosurgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access