Fig. 8.1.
Airway pressure curve at the end of inspiration and held expiration. Initial decrease in peak pressure followed by the plateau pressure.
An acute change in upper airway resistance (e.g., endotracheal tube obstruction) would generate an acute increase in Ppeak with unchanged plateau pressure.
Decreased lung compliance would increase both Ppeak and plateau pressure (e.g., atelectasis, pneumonia, pneumothorax) whereas an endotracheal cuff leak would decrease the Ppeak (Fig. 8.2).
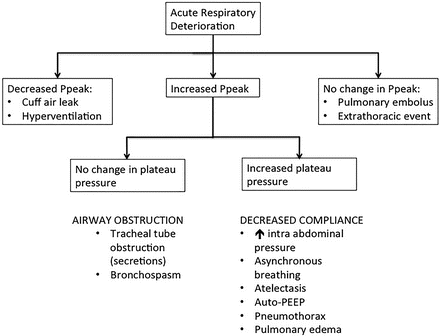

Fig. 8.2.
Assessment of acute respiratory deterioration of patients in the ventilator. Decreased lung compliance would increase both Ppeak and plateau pressure (e.g., atelectasis, pneumonia, pneumothorax) whereas an endotracheal cuff leak would decrease the Ppeak.
Mechanical breaths can be delivered by either pressure-cycled ventilation or volume-cycled ventilation.
Advantage of volume-cycled ventilation is that a constant volume is assured. However, in noncompliant lungs, intrathoracic pressures can be very high, which can result in lung injury (barotrauma) and have an adverse effect on cardiac output.
The increased intrathoracic pressure associated with mechanical ventilation can reduce ventricular filling by impeding venous return and reducing cardiac distensibility. Ventricular output is usually facilitated by the positive intrathoracic pressure. The end result in cardiac output and blood pressure will depend on which of the two effects predominates.
Modes of Mechanical Ventilation
Modes of mechanical ventilation are classified by the type of breath they deliver.
The ventilator settings can control, assist, or support the volume or pressure the clinician has determined to be delivered. Each breath is triggered either by the ventilator or the patient (Table 8.1) [1].
Mode
Breath strategy
Trigger
Type of breath
Mandatory
Assisted
Spontaneous
CMV
Volume or pressure cycled
Ventilator
Yes
No
No
AC
Volume or pressure cycled
Ventilator and patient
Yes
Yes
No
IMV/SIMV
Volume or pressure cycled
Ventilator and patient
Yes
Yes
Yes
PSV
Pressure-cycled
Patient
No
Yes
No
Some of the most frequently used modes are assist control (AC), synchronized intermittent mandatory ventilation (SIMV) and pressure support.
Controlled Mechanical Ventilation (CMV).
Minute ventilation is established by the respiratory rate and the tidal volume chosen by the practitioner.
The patient does not do any effort to trigger or assist with the respiration.
Assist Controlled (AC) Mode.
Clinician determines the minute ventilation by setting the respiratory rate and tidal volume.
The patient can increase the minute ventilation if she triggers a respiration.
Each patient-initiated breath receives the set tidal volume that has been established.
Synchronized Intermittent Mandatory Ventilation (SIMV).
Clinician determines the minute ventilation by setting the respiration rate and tidal volume.
Breaths are synchronized with patient’s inspiratory effort.
SIMV differs from AC in that the patient can also increase the minute ventilation by spontaneous breathing without ventilator assistance.
Initiation of Mechanical Ventilation
Consider the following parameters:
1.
Use of invasive or noninvasive mechanical ventilation.
2.
Mode of mechanical ventilation.
3.
Amount of support to be delivered.
4.
Initial ventilator settings.
The selection of the mode is generally based on clinician familiarity and institutional preferences [2]. Recent data suggests that lung-protective mechanical ventilation used for acute respiratory distress syndrome (ARDS) is safe and potentially beneficial in patients who do not have ARDS [3] (Table 8.2). The ideal level of respiratory support allows sufficient rest to the respiratory muscles, without causing atrophy, while attaining adequate ventilation. The AC mode delivers the highest level of support and can be used to initiate mechanical ventilation.
Table 8.2.
The principles of lung-protective ventilation.
1. Prevention of trauma due to excessive volume (tidal volume 4–8 mL/kg of PBW, with predicted plateau volume <30 cm H2O) |
2. Prevention of atelectasis by positive end-expiratory pressure (PEEP) ≥5 cm H2O and recruitment maneuvers (prolonged inspiration at 30–50 cm H2O for 30–40 s, a sigh breath with high tidal volume, high pressure during PSV) [3] |
3. Adequate ventilation (respiratory rate: 20–35) |
4. Prevention of hyperoxia (peripheral oxygen saturation SpO2 88–95 %) |
Management of Mechanical Ventilation After Initiation [4]
1.
Reduce tidal volume (Vt) by 1 mL/kg every 2 h until Vt = 6 mL/kg and measure plateau pressure. Continue reducing Vt until plateau pressure <30 cm H2O or Vt = 4 mL/kg.
2.
Monitor arterial blood gas and correct with respiratory rate and Vt settings as necessary.
3.
Goals: Vt = 6 mL/kg, plateau pressure <30 cm H2O and pH = 7.30–7.45.
Acute Respiratory Distress Syndrome (ARDS)
In 2011, The European Society of Intensive Care Medicine, endorsed by the American Thoracic Society and the Society of Critical Care Medicine, developed the Berlin definition for ARDS. It is described as an acute, diffuse, inflammatory lung injury leading to increased vascular permeability, increased lung weight, and loss of aerated lung tissue. Diffuse alveolar damage leads to increased dead space and decreased lung compliance. The clinical result is hypoxemia and diffuse bilateral radiographic opacities [5] (Table 8.3).
Acute respiratory distress syndrome | ||
|---|---|---|
Timing | Within 1 week of a known clinical insult or new worsening respiratory symptoms | |
Chest imaging | Bilateral opacities-not fully explained by effusions, lobar/lung collapse, or nodules | |
Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (e.g., echocardiography) to exclude hydrostatic edema if no risk factor present | |
Mild | 200 mmHg < PaO2/FiO2 ≤300 mmHg with PEEP or CPAP ≥5 cm H2O | |
Oxygenation | Moderate | 100 mmHg < PaO2/FiO2 ≤200 mmHg with PEEP ≥5 cm H2O |
Severe | PaO2/FiO2 ≤100 mmHg with PEEP ≥5 cm H2O | |
ARDS severity correlates well with mortality and with increased median duration of mechanical ventilation (Table 8.3) [5]. Mild, moderate, and severe ARDS are associated with 27, 32, and 45 % mortality, respectively.
Consensus exists about using mechanical ventilator strategies to decrease ventilator-associated lung injury in ARDS.
Low tidal volume ventilation (LTVV) is associated with decreased mortality and more days off the ventilator [6, 7]. Lung-protective ventilation principles should be employed (Table 8.2).
The Vt should be adjusted based on plateau pressure.
The plateau pressure should be measured every 4 h or after each change in PEEP or Vt.
The goal plateau pressure is <30 cm H2O.
Oxygenation goal during LTVV is a PaO2 between 55 and 80 mmHg or a SpO2 between 88 and 95 %. This is achieved by adjusting PEEP and FiO2.
To achieve these goals, permissive hypercapnia might be necessary.
The open lung ventilation approach uses LTVV combined with increased PEEP.
Weaning from the Ventilator and Extubation
Assess for safe discontinuation of the ventilator as soon as possible. Prolonged intubation can lead to increased complications (e.g., upper respiratory airway edema, infection) and costs. Balance risks of premature extubation, such as loss of airway, compromise of gas exchange, aspiration and respiratory muscle fatigue [10]. In order to prevent either early or late extubation, weaning trials have been established. Approximately 10–20 % of extubation attempts fail, despite successful weaning trials. Those patients have a higher mortality rate of 20–50 % [11].
Criteria for performing a weaning trial (Table 8.4):
Table 8.4.
Recommendations to wean mechanical ventilation.
Recommendations to wean mechanical ventilation |
|---|
Initiate weaning trial when: |
1. Resolution/improvement of the condition for which intubation is required 2. Cardiovascular stability without the need of vasopressors 3. No continuous sedation 4. Adequate oxygenation (PaO2/FiO2 ≥150 mmHg with PEEP ≤5–8 cm H2O, FiO2 ≤0.4–0.5) and pH ≥7.25 |
Perform daily spontaneous breathing trial (SBT) for 30–120 min |
Patient succeeds SBT if: |
1. Adequate respiration pattern 2. Adequacy of gas exchange 3. Hemodynamic stability 4. Subjective comfort |
Before extubation assess airway patency (cuff leak) and patient’s capacity to protect the airway (cough reflex) |
1.
Resolution/improvement of the condition for which intubation is required.
2.
Cardiovascular stability without the need of vasopressors.
3.
No continuous sedation.
4.
Adequate oxygenation (PaO2/FiO2 ≥150 mmHg with PEEP ≤5–8 cm H2O, FiO2 ≤0.4–0.5) and pH ≥7.25.
The weaning technique that has been shown to be the most successful is a daily spontaneous breathing trial (SBT) in which the patient is left to breathe through the T-tube for 30–120 min. During the SBT, minimal pressure support can be given to account for the resistance the T-tube generates.
Clinical signs and symptoms to monitor during SBT:
Frequency and depth of breathing.
Adequacy of gas exchange (arterial blood gas).
Hemodynamic stability (blood pressure, heart rate and respiratory rate).
The patient’s subjective comfort level.
Once the patient has passed the SBT, the decision to discontinue the endotracheal tube should follow the assessment of airway patency (cuff leak) and the ability of the patient to protect the airway (cough reflex).
The cuff leak evaluates the volume escaping between the endotracheal tube and the trachea as a predictor of airway obstruction after extubation:
With the endotracheal tube cuff inflated, the patient is placed in assist-control settings.
Aspirate the tube and the upper airway (evaluating cough reflex).
Measure the inspiratory and expiratory tidal volumes (read on the ventilator). These volumes are expected to be similar.
Next, the tidal inspiratory and expiratory volumes are measured with the cuff deflated. If the difference of these volumes is above 110 cc it should be interpreted as a patent airway and the negative predictive value for stridor after extubation approaches 98 % [12].
Management of Sepsis and Fluid Resuscitation
Sepsis
Sepsis is a complex clinical condition in which an infection induces a systemic inflammatory response. The landmark signs of sepsis are:
Systemic inflammation.
Vasodilation.
Leukocytosis.
Increased vascular permeability.
Sepsis can lead to multiple organ dysfunction syndrome (MODS), which has a high mortality rate. Adequate and timely treatment of sepsis is key to success of patient’s management. Early goal-directed therapy within 6 h of diagnosis decreases in-hospital mortality by more than 15 % [13].
The in-hospital mortality rate for sepsis is reported to be about 16 % [14]. Escherichia coli and MRSA infections are the most common sepsis-associated infections. Complication of device, implant or graft is the most common reason for sepsis-related hospitalization [14].
The progression to MODS from an infectious origin can be thought as a continuum from infection, bacteremia, sepsis, severe sepsis, septic shock to multiple organ dysfunction syndrome [15].
Systemic inflammatory response syndrome (SIRS) is characterized by dysregulated inflammation, which can be associated to noninfectious processes (e.g., pancreatitis, autoimmune disorders, vasculitis, burns). Sepsis and severe sepsis definitions and criteria can be found in Tables 8.5 and 8.6. Septic shock is defined as sepsis-induced hypotension refractory to adequate fluid resuscitation.
Table 8.5.
Diagnostic criteria for sepsis.
Sepsis-presence (probable or documented) of infection together with systemic manifestations of infection |
Systemic manifestations of infection • Fever (>38.3 °C) • Hypothermia (core temperature <36 °C) • Heart rate >90 min or more than two SD above the normal value for age • Tachypnea • Altered mental status • Significant edema or positive fluid balance (>20 mL/kg over 24 h) • Hyperglycemia (plasma glucose >140 mg/dL or 7.7 mmol/L) in the absence of diabetes • Leukocytosis (WBC count >12,000 μL) • Leukopenia (WBC count <4,000 μL) • Normal WBC count with greater than 10 % immature forms • Plasma C-reactive protein more than two SD above the normal value • Plasma procalcitonin more than two SD above the normal value |
Hemodynamic variables |
• Arterial hypotension (SBP <90 mmHg, MAP <70 mmHg, or an SBP decrease >40 mmHg in adults or less than two SD below normal for age) |
Organ dysfunction variables • Arterial hypoxemia (PaO2/FiO2 < 300) • Acute oliguria (urine output <0.5 mL/kg/h for at least 2 h despite adequate fluid resuscitation) • Creatinine increase >0.5 mg/dL or 44.2 μmol/L • Coagulation abnormalities (INR >1.5 or aPTT >60 s) • Ileus (absent bowel sounds) • Thrombocytopenia (platelet count <100,000 μL-1) • Hyperbilirubinemia (plasma total bilirubin >4 mg/dL or 70 μmol/L) |
Tissue perfusion variables |
• Hyperlactatemia (>1 mmol/L) |
• Decreased capillary refill or mottling |
Table 8.6.
Severe sepsis.
Sepsis-induced tissue hypoperfusion or organ dysfunction (any of the following thought to be due to the infection) |
• Sepsis-induced hypotension • Lactate above upper limits laboratory normal • Urine output <0.5 mL/kg/h for more than 2 h despite adequate fluid resuscitation • Acute lung injury with PaO2/FiO2 <250 in the absence of pneumonia as infection source • Acute lung injury with PaO2/FiO2 <200 in the presence of pneumonia as infection source • Creatinine >2.0 mg/dL (176.8 μmol/L) • Bilirubin >2 mg/dL (34.2 μmol/L) • Platelet count <100,000 μL • Coagulopathy (international normalized ratio >1.5) |
Management of Sepsis
Have a high index of suspicion, as early recognition is crucial to optimize patient outcomes.
Stabilize the airway.
Provide supplemental oxygen along with intubation for mechanical ventilation if necessary.
Assess perfusion through vital signs (blood pressure, heart rate, core temperature), mental status, urine output, temperature, and lactate level.
Correct physiologic abnormalities.
If infection is suspected, collect blood cultures and start broad-spectrum antibiotics.
Draw blood cultures prior to starting antibiotics, but do not delay the start of antibiotics for more than 45 min [15]. Delay in antibiotics initiation is a strong predictor of mortality [16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree