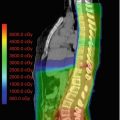
Fig. 16.1
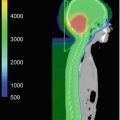
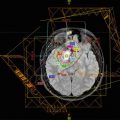
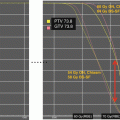
This example depicts a 16-year-old female who presented with headaches, visual changes, and amenorrhea. On MRI (a), she was found to have a suprasellar mass with radiologic changes consistent with craniopharyngioma. She underwent a subtotal resection as confirmed with postoperative imaging (b). She then received postoperative proton beam radiation therapy to a dose of 50.4 cGE in 28 fractions using a 3-field passive scatter technique (c, d)
Visual symptoms or endocrine abnormalities are more specifically attributable to suprasellar tumors, like craniopharyngiomas. The presentation and severity of symptoms range based on the tumor location. For instance, pressure on the optic chiasm may result in temporal quandrantanopias. However, a variety of visual symptoms are possible based on the particular growth pattern of the tumor and include: diplopia, blurred vision, decreased acuity, or in severe cases sometimes unilateral blindness. Visual symptoms tend to be the presenting sign more common in adults (~80%) than children (~20–60%) (Sughrue et al. 2010; Muller 2013).
Endocrine deficiencies are also common due to disruption of the hypothalamic-pituitary axis. At diagnosis, up to almost 90% of patients present with at least one hormone deficit, most commonly growth hormone (GH) (75%), followed by gonadotropins (40%), adrenocorticotropic hormone (ACTH) (25%), and thyroid-stimulating hormone (TSH) (25%) (Muller 2008; Caldarelli et al. 2005; Hoffman et al. 1992). Each hormone deficit has its own associated symptoms. For instance, GH deficiencies result in growth retardation and delayed bone age. Low gonadotropin levels interfere with pubertal changes, which may be more clinically apparent in adolescents. Low TSH levels result in hypothyroidism leading to fatigue, cold intolerance and weight gain (Rath et al. 2013; Rose et al. 1999).
Overall, there is a relatively specific constellation of symptoms in patients with suprasellar tumors. Therefore, patients presenting with a combination of headaches, visual impairment, and signs of endocrine abnormalities (decreased growth rate, short stature, or polydipsia/polyuria) should be evaluated for a craniopharyngioma (Muller 2010).
16.4 Radiographic Findings
While plain radiographs are rarely used for diagnosis of this disease, several classic findings can be seen on these films. First, most patients have sellar abnormalities, including enlargement (~65%) or erosion (~44%) (Moore 2000). Second, tumor-associated calcifications can also be seen on plain radiographs, which are more common in children (up to 90%) due to the preponderance of the adamantinomatous histology (Warmuth-Metz et al. 2004).
A computed tomography (CT) scan is commonly the initial imaging obtained for workup of patients with the previously discussed symptomatology. On CT, the tumors are characteristically described as suprasellar and lobular with a central solid component surrounded by multiple, various sized, hypodense cysts. Calcifications are also best demonstrated by CT (Warmuth-Metz et al. 2004). Additional imaging is typically performed using magnetic resonance imaging (MRI); this is particularly useful for providing detailed information related to the anatomic relationship of the tumor to nearby structures. On T1-weighted images, the solid tumor component (appears isointense without contrast) and cyst rims typically enhance with contrast and the cyst fluid may appear hypo-intense. Conversely, on the T2-sequence, the tumor will typically appear hyperintense.
16.5 Workup
Initial assessment for craniopharyngiomas usually consists of obtaining adequate neuroimaging with CT brain and subsequent MRI, as discussed above. Features such as calcifications and cystic regions often help to narrow the differential diagnosis, making other lesions such as germ cell tumors less likely. Depending on the acuity and need for neurosurgical intervention to manage hydrocephalus, additional workup is warranted.
Ideally, patients with tumors that are suspicious for craniopharyngioma should receive evaluation and management by a multidisciplinary team with subspecialists from neurosurgery, radiation oncology, medical oncology, endocrinology, ophthalmology, and neuropsychology. A baseline visual acuity exam is useful in determining where there is optic pathway compression and for establishing baseline function. Additionally, since most patients have at minimum partial hypopituitarism, a complete endocrine assessment is important, especially in order to identify adrenal or thyroid dysfunction prior to surgery. Finally, despite their relatively unique appearance, imaging alone does not suffice to establish the diagnosis of craniopharyngioma, and histologic confirmation in required (Moore 2000; Sughrue et al. 2010; Muller 2010).
16.6 Treatment
The optimal therapeutic strategy for craniopharyngiomas remains controversial. The two basic approaches include either an aggressive surgical resection that attempts a gross total resection (GTR) versus a more conservative surgery followed by radiation therapy to treat residual disease. Unfortunately, the published literature and lack of randomized data have not been able to resolve the debate. Notably, biases against either strategy are based primarily on morbidities associated with historical cohorts. For instance, significant advances in neurosurgical techniques have decreased the morbidity of resections and perhaps more aggressive approaches are warranted. Similarly, radiation therapy techniques have evolved rapidly allowing for more accurate, conformal dose deposition while minimizing radiation to nearby critical structures. Therefore, it is imperative that an experienced multidisciplinary team evaluate patients with craniopharyngiomas tumors for personalized and optimal treatment planning.
16.6.1 Surgery
Surgery is indicated in all patients in order to achieve a histologic diagnosis, allow for cyst or hydrocephalus decompression, and to minimize disease burden (Van Effenterre and Boch 2002). Some surgeons believe that an aggressive GTR is required for cure, while others believe the morbidity associated with that approach is too great and opt for cyst drainage and a subtotal resection (STR) with planned postoperative radiation therapy (Aggarwal et al. 2013; Fahlbusch et al. 1999; Merchant et al. 2002; Sanford 1994; Stripp et al. 2004; Weiner et al. 1994; Yasargil et al. 1990; Clark et al. 2013; Schoenfeld et al. 2012).
Initial surgical intervention is focused on relieving acute symptoms related to these tumors. In patients presenting with hydrocephalus, decompression of the lesion itself is the favored treatment approach to restore CSF flow (Fahlbusch et al. 1999; Choux and Lena 1979). However in severe cases, an external drain may be required to relieve pressure prior to tumor resection.
The approach to surgical resection is dependent on the location and makeup of the tumor. Historically, a common surgical approach included a right frontotemporal incision (Fahlbusch et al. 1999). Also, suprasellar tumors were also resected using a transcranial approach, while prechiasmatic tumors may be best visualized via a supraorbital craniotomy (Moore 2000). For tumors that are primarily intra-sellar, an endoscopic, transsphenoidal approach may provide optimal visualization while remaining minimally invasive (Elliott et al. 2011; Zona and Spaziante 2006). More commonly, newer techniques including microsurgery, endoscopic assistance, and minimally invasive approaches have allowed neurosurgeons to improve the quality of their resections while minimizing morbidity (Sughrue et al. 2010; Muller 2008, 2013). Ultimately, the intraoperative findings will dictate the degree of resection.
16.6.2 Radiation Therapy
Factors, which most impact the choice of whether to proceed with adjuvant radiotherapy or observation, are most often the presence of residual disease and the age of the patient. For tumors arising in very young patients, a discussion is warranted regarding the appropriateness of observation since very young children are particularly susceptible to adverse radiation effects. However, because incompletely resected lesions have a high rate of local relapse without adjuvant therapy (71–90%), postoperative radiotherapy should be recommended in patients with subtotal resection (Clark et al. 2013; Becker et al. 1999).
Numerous radiation therapy techniques exist and have been utilized with excellent outcomes in terms of disease control for these tumors. It is, however, worth noting that no prospective studies evaluating the benefits of advanced technology exist, in terms of reduced radiation adverse effects, either for photon or proton therapy. In the majority of published literature, photon-based therapies, both traditionally fractionated and hypo-fractionated stereotactic techniques have been employed (Merchant et al. 2002, 2006; Becker et al. 1999; Habrand et al. 1999, 2006; Minniti et al. 2009). Merchant and colleagues conducted a single arm prospective study of reduced margin 3D conformal radiation therapy for pediatric patients with craniopharyngioma (Merchant et al. 2006). In a total of 28 patients, the solid and cystic tumor components along with a 1 cm expansion were targeted to a dose between 54 and 55.8 Gy with acceptable rates of disease control. Greenfield and colleagues have also retrospectively evaluated the use of intensity modulated radiation therapy techniques (Greenfield et al. 2015). These investigators documented high rates of disease control but also noted a high burden of pre-radiotherapy comorbidities including endocrinopathies. More recently, dosimetric studies of volumetric arc therapy (VMAT) have been conducted and indicate that close attention to beam angles is important in order to minimize hippocampal exposure (Uto et al. 2016).
In addition to studies using common 1.8 Gy fractionation schemes, given the relatively well-demarcated tumor boundaries on imaging, radiosurgery, or hypo-fractionated stereotactic radiotherapy have also been explored in the treatment of these tumors. For radiosurgery, the most commonly employed platform has been gamma knife. Patients selected for gamma knife radiotherapy typically have small tumors, measuring less than 3 cm; also, there must be a safe separation (generally 3–5 mm depending on stereotactic delivery technique) between the target and nearby critical structures such as the optic chiasm in order to allow for adequate target coverage while respecting normal tissue constraints. Reported doses delivered in single fractions using gamma knife range from 12 to 14 Gy (Amendola et al. 2003; Kobayashi 2009; Mokry 1999; Chung et al. 2000). In these select patients, reported disease control rates seem acceptable and toxicities minimal (Lee et al. 2014; Park et al. 2011; Xu et al. 2011). However, patient numbers included are small and outcomes for adult and pediatric patients are frequently not separated. Given various differences in patient selection (i.e., tumor volumes, etc.), it is difficult to compare these results to traditional fractionated regimens.
As for many pediatric brain tumors, proton therapy is increasingly used in the treatment of craniopharyngiomas. Boehling and colleagues described in detail the dosimetric advantages of proton therapy including the potential for sparing of vascular structures and the hippocampus, especially with advanced proton therapy delivery modalities, namely intensity modulated proton therapy (IMPT) (Boehling et al. 2012). One of the preliminary reports by Luu and colleagues on the use of proton therapy for patients with craniopharyngiomas showed equivalent local control rates (88%) as compared to previous photon-based studies (Luu et al. 2006). More recently, a multi-institutional study comparing proton therapy to photons, again reported comparable outcomes (Bishop et al. 2014).
16.7 Radiation Details
Currently, for traditionally fractionated therapies, doses range from 50.4 to 54 Gy delivered at 1.8 per fraction. The commonly used upper limit of 54 Gy likely represents the tolerance for structures such as the optic chiasm, which are frequently immediately adjacent to the target (Merchant et al. 2002, 2006; Bishop et al. 2014; Kiehna and Merchant 2010). Advanced photon-based therapies have improved normal tissue sparing, which includes the use of intensity modulated radiation therapy (IMRT) (Greenfield et al. 2015; Merchant et al. 2013). In a single institution series, 24 patients were treated to a dose of 50.4 Gy using IMRT, and the 5- and 10-year progression-free survival rates were consistent with those seen with 3D conformal or other photon-based therapies (Greenfield et al. 2015). Regarding target delineation, the gross tumor volume (GTV) is typically easily visualized and delineated following fusion of MR imaging with treatment planning CT. While there is some discussion of whether or not all cystic areas should be included in the GTV, tumor cells could be present in the cyst walls, which is the reason to ensure coverage with the prescription isodose line.
Some debate surrounds the appropriate clinical target volume (CTV) expansion to be utilized. Historically, larger expansions of 1–2 cm have been employed given less reliable immobilization and image-guidance. However, more recently, studies have suggested that a smaller target volume expansion of 5 mm may be safely employed (Merchant et al. 2013). This is justifiable based on the fact that craniopharyngiomas are not inherently infiltrative, unlike gliomas or other intrinsic brain tumors. Preliminary studies reporting disease control outcomes utilizing reduced margin treatments have documented good disease control outcomes (Merchant et al. 2013). However, it is important to note that the CTV should be customized for each individual patient; this includes ensuring coverage of areas of adhesion noted intraoperatively. For photon therapy planning target volume (PTV), expansions will depend on institutional practices with common values falling between 3 and 5 mm.
16.7.1 Cystic Considerations for Radiation Planning
While it has long been known that craniopharyngiomas may contain cystic components, only recently has it been appreciated the dynamic changes that occur within these structures. Investigators at the Massachusetts General Hospital were among the first to describe in detail the potential for rapid cystic changes in these tumors (Winkfield et al. 2009). A group of 24 pediatric patients, 19 with a cystic component, underwent treatment. Seventeen of the 19 patients with cysts received surveillance imaging during the course of radiation therapy. Six of these patients had imaging evidence of cyst enlargement with 4 requiring revision of the radiation plan in order to ensure target coverage. The authors recommended routine imaging during the course of radiation therapy in patients with tumors containing cystic components (Winkfield et al. 2009). Following this initial observation, a subsequent report from the University of Texas MD Anderson Cancer Center confirmed relatively frequent cystic changes during irradiation (40%), with some patients requiring alteration to the treatment plan (20%) (Bishop et al. 2014). Therefore, when small CTV and PTV expansions are used, weekly or biweekly imaging during treatment is recommended in patients with cystic components to their tumors in order to ensure adequate tumor coverage. Additionally, close surveillance imaging is of greater importance in the setting of reduced CTV margins and/or the use of proton therapy.
16.7.2 Intracavitary Therapies
A less commonly employed treatment approach is the intracavitary injection of either β-emitting radioisotopes or sclerosing substances. The radioisotopes (Schoenfeld et al. 2012) phosphorus or 90yttrium have been used both as a primary and salvage treatment for cystic craniopharyngiomas, in which they are injected into the cysts via a catheter. Once within the cyst, photons are emitted by beta decay allowing for delivery of high doses of radiation to the epithelial lining of the cyst. Several studies have reported cyst regression in 81–88% of patients (Blackburn et al. 1999; Leksell 1952; Pollock et al. 1995). Alternatively, intracystic catheters can be used to instill chemotherapeutic agents (i.e., bleomycin or interferon α) with some efficacy (Cavalheiro et al. 2010; Schubert et al. 2009).
16.8 Outcomes
There are not evidence-based guidelines or recommendations regarding a follow-up plan for patients with a history of craniopharyngioma. However, given the potential for cystic changes following treatment, a best practices approach would suggest that neuroimaging should be obtained routinely. Our institutional approach is to obtain post-radiation imaging 4–6 weeks following completion of treatment and every 3 months thereafter for 2–3 years, at which point longer intervals may be recommended. In addition to monitoring for disease relapse, a multidisciplinary team is essential to manage disease-related and iatrogenic toxicities. Endocrine function will need to be closely monitored and supplements added as indicated, especially for developing children and adolescents; the importance of close endocrine follow-up cannot be overstated. Periodic visual field testing is also important for early intervention. Other members of a multidisciplinary team (internists/pediatricians, neuropsychologists, teachers, dieticians, etc.) may be required depending on the needs of the patient.
16.8.1 Outcomes
While disease control rates are high, craniopharyngiomas are associated with decreased overall survival compared to the normal population; the 10-year survival outcomes commonly reported are between 80 and 91% (Schoenfeld et al. 2012; Bishop et al. 2014; Merchant et al. 2013; Karavitaki et al. 2006; Pereira et al. 2005; Fernandez-Miranda et al. 2012). In addition to the deaths attributable to disease progression or surgical mortality, these patients are at higher risk of cardiovascular and cerebrovascular death (Pereira et al. 2005). Notably, based on the current evidence, there does not seem to be a difference in long-term survival between patients treated with GTR versus STR and postoperative radiation therapy. However, a recent SEER study did suggest a short-term survival benefit independently associated with STR (HR 0.39) and RT (HR 0.45) on multivariate analysis (Zacharia et al. 2012).
In terms of surgical resection, GTR rates range widely (between about 30 and 75%), with recurrences most commonly reported to occur in about 8–30% of patients following a GTR (Caldarelli et al. 2005; Fahlbusch et al. 1999; Sanford 1994; Stripp et al. 2004; Shi et al. 2008; Gardner et al. 2008). Conversely, the rate of recurrence following a STR alone is unacceptably high (40–75%) without RT (Fahlbusch et al. 1999; Karavitaki et al. 2005; Villani et al. 1997). However, direct outcome comparisons are difficult given the variable median follow-up and differing definitions of disease progression.
Patients that receive STR and postoperative radiation therapy appear to have similar control rates to GTR with disease relapse occurring in 0–12% of patients (Schoenfeld et al. 2012; Bishop et al. 2014; Merchant et al. 2013). Several studies have also differentiated solid from cystic tumor progression. Immediately following radiotherapy many patients experience transient cyst growth before they regress (Bishop et al. 2014; Shi et al. 2008). Shi and colleagues reported cyst enlargement in 11 of 21 patients following radiation therapy. Similarly, Bishop and colleagues observed a third of patients to have evidence of cyst growth within 3 months of completing treatment. However, in the majority of cases, growth was transient and followed by subsequent shrinkage of the cystic component. Reported 3-year recurrence rates of the solid tumor component were 5% versus 24% for cysts (Bishop et al. 2014). Cyst growth is appreciably more challenging to control, but its biologic significance is undefined.
If tumors recur, management is increasingly difficult primarily because surgical resection of recurrent tumors is more challenging. The recurrent tumors are commonly more adherent to surrounding tissue with poorly defined tissue planes, which is evidenced by the lower rate of resection (13–53%) achieved at the time of recurrence (Fahlbusch et al. 1999; Villani et al. 1997; Duff et al. 2000). For patients that recur, surgery alone is unlikely to provide durable control. In a study by Kalapurakal and colleagues, the 10-year progression-free survival after surgery and radiation therapy for recurrent tumors was 82–83% compared to 0% for surgery alone (Kalapurakal et al. 2000). However, withholding radiation in the definitive treatment setting in order to reserve it for use at the time of salvage is not justified based on available evidence. In fact, a recently published study by Bishop and colleagues showed that recurrent cyst growth was associated with poorer visual outcomes and hypothalamic obesity; furthermore, radiation therapy as salvage therapy negatively affected endocrine function (Bishop et al. 2014). Therefore, reserving radiation for salvage in the setting of STR is not advisable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree