Cranial Radiosurgery
Frank J. Bova
Development
In the mid-twentieth century, the advent of Cobalt teletherapy units, and subsequently linear accelerators, helped radiation therapy play an increasingly important role in cancer treatment. During this time, external beam radiation therapy relied heavily upon the enhanced ability of normal cells over that of cancer cells to repair sublethal radiation damage. A basic schema was developed where a course of therapy would be divided into small fractions, each delivering a sublethal dose of radiation to a specified target volume. In the time interval between therapeutic fractions, the normal tissues would better repair radiation damage than would the cancerous cells, so that by the end of a course of treatment, the targeted cancer cells would have amassed significantly more radiation damage than would normal cells receiving identical doses (Fig. 15.1).
This delivery technique was necessary for two reasons: the first being that in the mid-twentieth century, neither computed tomography (CT) nor magnetic resonance imaging (MRI) was available, limiting the clinician’s ability to map out and plan three-dimensional (3D) conformal radiation distributions. The second, which still exists today, is that the relative ratio of normal tissue cells to cancer cells varies considerably through the volume being targeted by the therapeutic radiation beams. This ratio begins at the site of the primary tumor with a high proportion of cancer cells to normal tissue cells, moves to a more diffuse concentration of cancer cells at the tumor’s margin, and finally concludes with the lowest concentration of cancer cells, as regions of suspected microscopic disease are included in the targeted volume. It was necessary to develop a therapeutic tool that resulted in more cancer cell death relative to normal tissue cell death for a given course of therapy across this spectrum of tumor burden.
Radiosurgery, defined as a single-fraction stereotactically targeted radiation therapy, proposed a paradigm shift in the art of radiation delivery. This new approach would not attempt to leverage differential normal cell to cancer cell repair of sublethal damage, but instead deliver a highly concentrated dose of radiation exclusively to the volume of highly concentrated tumor cells. The normal tissue cells would be spared as a result of a very steep dose gradient, significantly reducing the dose to normal tissues.
The term “radiosurgery” was initially conceived by a neurosurgeon, Lars Leksell (1). Leksell’s first attempt at a delivery scheme was to provide a concentrated radiation dose by the attachment of an x-ray tube onto an arc-centered stereotactic head-frame system designed to allow a target to be placed at the center of two orthogonal arc systems. The system was provided with a probe holder to point toward the intersection of the two arcing planes. The probe holder could then be moved along either arc while maintaining a trajectory that pointed to the target. Mounting an x-ray tube in place of the probe holder provided a method of delivering a radiation beam that would remain focused on the target. This, in turn, provided delivery of multiple non-coplanar beams, with separate entrance and exit pathways, that all intersected over the target.
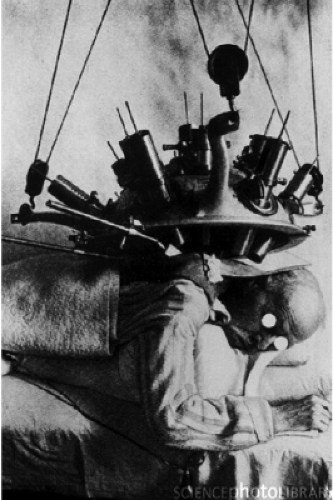
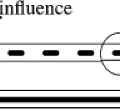
In the 1950s, teletherapy was still in its infancy with the transition underway from delivery with x-ray tubes and radium systems to Cobalt-based units. Leksell undoubtedly had a thorough knowledge of state-of-the-art radiation delivery devices. Due to the limited specific activity and the resultant self-shielding, tele-radium sources had a very low dose rate, leading to extended treatment times. Novel approaches at increasing the dose rate were being pursued. One such approach was to develop a device that could simultaneously focus multiple radium sources at a target (Fig. 15.2) (2). These tele-radium beams were focused at a specific depth, converging at a point in space and then diverging as they left the target volume. This geometric-focusing technique is very similar to Leksell’s approach used first in his arc-mounted orthovoltage x-ray tube design and then in his 179 Cobalt-60 source GammaKnife design (3,4). Leskell’s primary contribution to radiation delivery was the realization that the coupling of a multi-focused radiation beam delivery system to a stereotactic reference system could enable a highly focused, high dose of radiation to be delivered to a defined target while providing significant sparing of adjacent normal tissues, a development that preceded isocentric teletherapy designs.
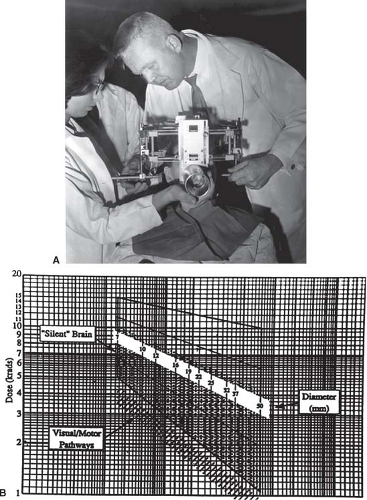
Prior to the development of the GammaKnife unit, in separate efforts, Leksell and Kjellberg had adapted fixed-port proton-beam units for stereotactic radiosurgical applications (Fig. 15.3) (5,6). These pioneering systems treated significant numbers of patients and provided early data on appropriate clinical doses for malignant, as well as
benign, targets. The fact that these units were retrofitted for patient application and not readily available to the therapeutic community at large limited their widespread application. Leksell’s development of the self-contained GammaKnife provided the first practical, commercial unit to offer dose distributions that rivaled the dose concentration and dose gradient of particle beam therapy (Fig. 15.4). While Leksell’s initial intent was to provide a means to produce functional lesion, the anatomic atlases available in the 1950s proved insufficient as the primary image guidance and targeting tools and as mentioned CT and MRI, the tools required for such a target definition, would not be available for several decades.
benign, targets. The fact that these units were retrofitted for patient application and not readily available to the therapeutic community at large limited their widespread application. Leksell’s development of the self-contained GammaKnife provided the first practical, commercial unit to offer dose distributions that rivaled the dose concentration and dose gradient of particle beam therapy (Fig. 15.4). While Leksell’s initial intent was to provide a means to produce functional lesion, the anatomic atlases available in the 1950s proved insufficient as the primary image guidance and targeting tools and as mentioned CT and MRI, the tools required for such a target definition, would not be available for several decades.
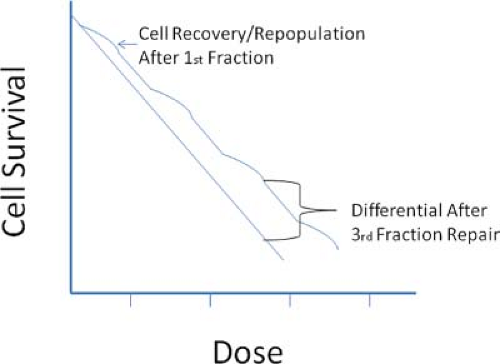 Figure 15.1. Figure shows the effects of a single-dose therapy versus that of a fractionated therapy. After each fraction, cells repair sublethal damage before the next fraction is administered. |
By the 1980s, techniques for CT-based stereotactic frame localization and subsequent MR–CT image fusion techniques began to provide a novel solution to the problems of 3D target definition (7). Combining these imaging techniques with a new dose computation algorithm allowed a paradigm shift in the treatment of intra-cranial targets. For the first time, the ability to deliver a high dose of radiation that was conformal to a 3D target shape and provide an exceedingly steep dose gradient along the entire target-to-normal tissue interface would become widely available to clinicians.
Over the past two decades, radiosurgery has gone from a novel treatment approach limited to a few academic centers to a treatment modality available in most communities. Systems capable of delivering these precise conformal doses with steep gradients have been developed on multiple platforms. Joining the isotope-based GammaKnife is a multitude of linear accelerator-based systems. These include traditional gantry-based linac approaches as well as robotic arm-mounted systems. These newer devices can provide for not only intra-cranial but also extra-cranial radiosurgical treatments. While each of these delivery platforms presents unique challenges, the underlying principles for targeting and the desired characteristics of the prescribed dose distributions remain the same.
Primary Objectives
The combination of stereotactic localization and the ability to produce a highly focused dose distribution with exceedingly high gradients provided a radical change from the existing fractionated treatment paradigm. This new imaging/treatment technique could successfully address intracranial targets in a single-fraction therapy, eliminating the dependence upon the ratio of radiation repair in normal tissue cells versus cancerous cells. This paved the way for clinicians to think of radiation as a tool of target elimination, which could be delivered in a single therapeutic event, similar to a surgical approach.
Radiosurgery allowed ionizing radiation to be applied to targets previously resistant to fractionated therapy. Dose distributions with an ever-evolving set of tools for high conformality and with exceedingly steep dose gradients provided new optimization parameters that were effectively leveraged against both benign and malignant targets. Radiosurgery treatment of arterial venous malformations is an example: when differential repair did not provide a sufficient therapeutic advantage relative to normal tissues, single-fraction conformal distributions with high dose gradients provided an effective therapy.
It is difficult to separate the effects of conformality and gradient on the success of radiosurgery treatments. The design of the first dedicated radiosurgery tool, the GammaKnife, allowed the treatment of spherical and irregular targets with a planning/delivery tool, commonly referred to as “sphere packing.” This technique uses a class solution
to produce a highly concentrated sphere of dose with very steep dose gradients. For round targets, a sphere is simply fitted to the target volume. For nonspherical targets, the technique fits the largest possible sphere inside the target, removes the volume covered by that sphere, and repeats the process with the remaining volume. The result of this process is an alignment of the target–normal tissue interface with the dose-sphere’s steep gradient. The result is a high degree of conformality while maintaining a high dose gradient along the entire target’s surface.
to produce a highly concentrated sphere of dose with very steep dose gradients. For round targets, a sphere is simply fitted to the target volume. For nonspherical targets, the technique fits the largest possible sphere inside the target, removes the volume covered by that sphere, and repeats the process with the remaining volume. The result of this process is an alignment of the target–normal tissue interface with the dose-sphere’s steep gradient. The result is a high degree of conformality while maintaining a high dose gradient along the entire target’s surface.
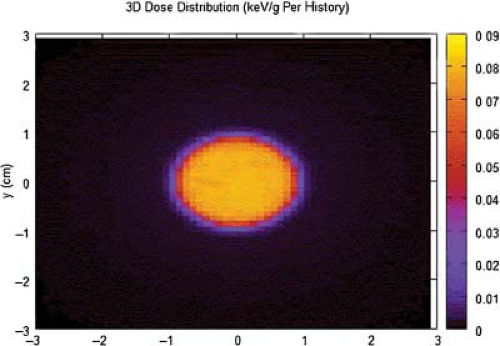 Figure 15.4. GammaKnife with dose distribution for an 18-mm helmet. The distribution and gradient is relatively symmetric about the vertical axis of the displayed dose distribution. |
Early radiosurgery literature was based on particle beam treatments. For these treatments the Bragg peak provided a delivery tool that allowed both high conformality and little exit dose. The dose distribution provided by such a beam allows a smaller integral dose to be delivered. However, for many targets treated with proton radiosurgery, the effectiveness of the approach has not been shown to out-perform photon beam radiosurgery.
Although eliminating normal tissue from the prescription volume is generally accepted as a necessary parameter for an effective therapy with low complications, a high dose gradient has not been universally recognized as an equally important parameter. When radiosurgery gradients are examined, special attention is paid to the portion of the distribution where the dose decreases from the prescribed target dose to one-half the target dose (6,8,9,10). As the volume of treated target increases, the volume contained in the rim of normal tissue rapidly expands. Therapies for both malignant and benign diseases have shown limitations on the maximum size of a single fraction’s efficacy with targets. As the volume of the target increases, the dose that can be safely delivered decreases. It is thought that this expansion of the high-dose rim is at least partially responsible for the dose volume limit placed on radiosurgery treatments.
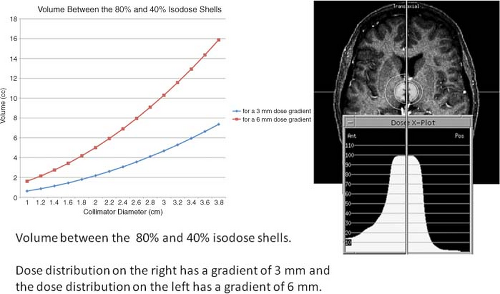
The importance of gradient can be appreciated when the volume enclosed in the high dose gradient, a shell defined by the edge of the prescribed isodose volume to one-half the treatment volume, is examined. The first evidence of a volume-limiting normal tissue threshold was the safe dose threshold versus target size published for particle beams (6). This curve demonstrates the relationship between complications and increasing target volume. Many other reports have provided clinical evidence demonstrating the relationship of increasing volumes being associated with increasing complications. Several reports have associated an increase in the 12-Gy volume to correlate with an increase in complications. As can be seen in Figure 15.5, the high-dose shell exposing normal tissue exponentially increases in volume as the target linearly increases. The lower curve in Figure 15.5 demonstrates the increase in this shell’s volume if the steep dose gradient, defined as the volume between the prescribed isodose surface and the isodose surface of one-half the prescribed dose, is maintained at 3 mm. The upper curve is the volume of this shell if the gradient is allowed to degrade from 3 to 6 mm. The net effect of the lower dose gradient is that the limiting dose volume is reached at smaller target volumes. For example, assume that it is safe to expose a rim of normal tissue, 2.0 cc in volume, to a gradient that is decreasing from 20 to 10 Gy. If a plan has a high dose gradient, 3 mm, as described above, this volume will not be reached until targets of ∼4 cc (2.0 cm average diameter) are treated. However, if the high dose gradient is allowed to degrade to 6 mm, then the 2.0-cc rim of normal tissue volume is reached when a target of only 0.9 cc (1.2 cm average diameter) is treated. Paying careful attention to the high dose gradient is critical to the delivery of a safe and effective therapy.
Imaging: Angiography
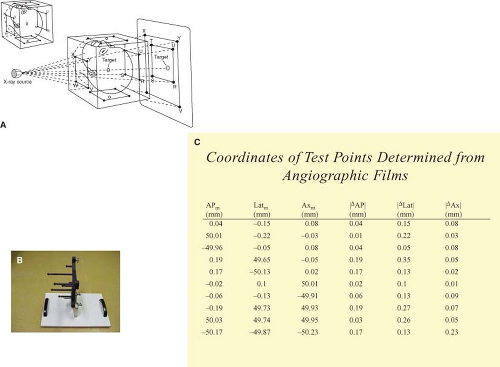
To provide a highly conformal treatment with steep dose gradients, the system must be able to provide a spatially accurate description of the tissues to be targeted. While suffering from a lack of true 3D target descriptions, plane film fiducial-based systems can provide the position of a point within a stereotactic reference frame to within a few tenths of a millimeter (Fig. 15.6). The overly defined fiducial system and solution, as described by Siddon, not only provides high-precision spatial accuracy, but also removes the previously required orthogonal geometry (11). In the late 1980s and early 1990s, images used to define intracranial
targets, such as arteriovenous malformations (AVMs) were obtained on plane film x-rays. These systems utilized flat-imaging planes that in turn provided the clinician with spatially undistorted projections. The temporal resolution was limited to the speed at which film changers could shuffle film in and out of cassettes, approximately two images per views per second. As x-ray film gave way to the higher speed image intensifiers, these unwarped projections were lost (Fig. 15.7). The image intensifier’s distortions were not only complex in any single orientation, but could vary nonlinearly with the orientation of the image intensifier. With the adoption of solid-state detectors, these projections are again presented without spatial distortion.
targets, such as arteriovenous malformations (AVMs) were obtained on plane film x-rays. These systems utilized flat-imaging planes that in turn provided the clinician with spatially undistorted projections. The temporal resolution was limited to the speed at which film changers could shuffle film in and out of cassettes, approximately two images per views per second. As x-ray film gave way to the higher speed image intensifiers, these unwarped projections were lost (Fig. 15.7). The image intensifier’s distortions were not only complex in any single orientation, but could vary nonlinearly with the orientation of the image intensifier. With the adoption of solid-state detectors, these projections are again presented without spatial distortion.
Simple orthogonal image sets are not capable of providing 3D descriptions of such vascular targets. Figure 15.8 shows a series of objects for which the orthogonal projections do not provide the information required for true 3D reconstructions. Although such views have been utilized for decades to reconstruct implanted radiation sources, the vascular images differ in that unique points in each projection cannot be matched. While these solid-state imaging systems provide clinicians with the perception of the 3D nature of the vasculature, the systems have not attempted to format and map this data relative to a stereotactic reference system.
Although angiography remains the gold standard for detection of most vascular targets, due to the above limitations, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have become the stereotactic targeting modalities of choice. This has resulted in many commercial stereotactic systems, abandoning radiographic fiducial frames. Newly introduced imaging platforms capable of rapid rotation during angiographic image acquisition may begin to compete with MRA and CTA as viable stereotactic imaging modalities. However, due to the unavailability of the required fiducial systems, it will be difficult for this mode of targeting to once again gain a clinical foothold.
Imaging CT
The first significant advance in the ability to define a true 3D intracranial target volume relative to a rigid reference system was the CT fiducial rod system (12,13). The addition
of the CT fiducial reference to a stereotactic frame provided a means by which imaged target tissues could be accurately and precisely mapped to a stereotactic reference platform. The stereotactic frame not only provided a method of analyzing each CT slice, providing a method of mapping CT pixels to stereotactic-based voxels (14), but, by analyzing the rods on each image, also provided a framework for image by image quality assessment. These systems removed scanner-dependent parameters from the required image analysis and subsequent mapping. No longer was it necessary to obtain an individual image’s relationship based on the CT table’s index or the scanner’s alignment to the stereotactic frame. All such parameters could now be derived from information contained in each individual CT image.
of the CT fiducial reference to a stereotactic frame provided a means by which imaged target tissues could be accurately and precisely mapped to a stereotactic reference platform. The stereotactic frame not only provided a method of analyzing each CT slice, providing a method of mapping CT pixels to stereotactic-based voxels (14), but, by analyzing the rods on each image, also provided a framework for image by image quality assessment. These systems removed scanner-dependent parameters from the required image analysis and subsequent mapping. No longer was it necessary to obtain an individual image’s relationship based on the CT table’s index or the scanner’s alignment to the stereotactic frame. All such parameters could now be derived from information contained in each individual CT image.
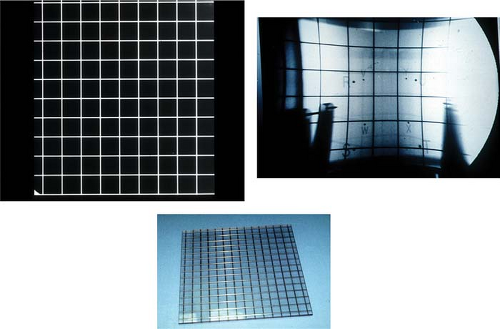 Figure 15.7. Lower image shows the lead grid used for imaging. Image in upper left shows the result of imaging the grid with a film system. Image on right shows the warped images resulting from the imaging with an image intensifying system.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|