Country
Currency
Year
Annual cost (billion)
Notes
Australiaa
AUD ($)
2005
56.6
$2,788 per individual
Australiab
AUD ($)
2010
88.9
Brazilc
USD ($)
2010
2.1
Including overweight costs
Canadad
CAD ($)
2006
11.0
Including indirect costs
Canadae
CAD ($)
2008
7.1
Including comorbid disease
Canadae
CAD ($)
2008
4.6
Without comorbid disease
Scotlandf
GBP (£)
2008
457
Including indirect costs
United Kingdomg
GBP (£)
2002
0.991–1.124
Additional 2.4–2.7 billion indirect costs
United Statesh
USD ($)
2008
147
United Statesi
USD ($)
2012
190.2
The United States remains the most expensive country to receive medical care, and has the highest expenditures for obesity management. As of 2012, the United States draws on 21 % of their healthcare costs to manage obesity (Cawley and Meyerhoefer 2012). In 2006, obesity expenditures were estimated to be 4.1 % of Canadian health expenses (Anis et al. 2010). Obese Americans were said to cost $1,429 USD more for healthcare than normal weight individuals (Cawley and Meyerhoefer 2012). Additionally, a United States report determined that by 2018, $344 billion would be spent on healthcare costs to manage obesity (Thorpe 2009).
2.2.3 Cost-Effectiveness of Bariatric Surgery
Globally, more than 340,000 bariatric procedures are performed annually, with one-third of those procedures performed in the United States. In Canada alone, an estimated 6,000 bariatric surgeries were performed in 2012, representing a 280 % increase in 6 years (Canadian Institute of Health Information 2014). These surgeries cost the Canadian healthcare system approximately $48 million in 2012. In the Canadian province of Alberta, the cost of laparoscopic adjustable gastric band (LAGB), laparoscopic sleeve gastrectomy (LSG), and laparoscopic Roux-en-Y gastric bypass (LRYGB) is $10,500, $12,000, and $18,000 CAD, respectively. The total rate for early and late complications is 12.4 %. Across Canada, early to intermediate complication rates are 5.3 % (Canadian Institute of Health Information 2014). An average of $475 CAD per patient is spent managing postoperative complications, including band removal, ulceration, hemorrhage, staple line leak, anastomotic stricture, and internal hernia. These patients also attend a multidisciplinary clinic, in preparation for surgery, attributing an additional $500 CAD cost. In total, Canadian bariatric surgery can cost $11,475–$18,975 CAD per patient (Sheppard et al. 2013, 2014a, b). The average cost of bariatric surgery within the United States is significantly more expensive at $24,000 USD (Mehrotra et al. 2005; Cremieux et al. 2008).
Regardless of a front-loaded cost of $10,000–$25,000, bariatric surgery has been established as a cost-effective strategy for treating obesity. Bariatric surgery reduces comorbidity management costs by more than half, and monthly savings of $900 USD per patient between 13 and 24 months (Cremieux et al. 2008; Sussenbach et al. 2012). Postoperative pharmaceutical savings of $180 USD/month can be expected (Monk et al. 2004). In Scotland, a noticeable decrease of 40 % in total pharmaceutical costs was seen, 24 months after bariatric surgery. The pharmaceutical cost for managing diabetes alone decreased by 78 % (£4,500–£1,000). Both hospitalization and medical services significantly decreased in cost after surgery (Karim et al. 2013).
Cost-effectiveness is measured by calculating the incremental cost effectiveness ratio (ICER), which contrasts incremental costs with incremental health benefits (increased years of life). When comparing health interventions (e.g., surgery vs. nonsurgical management of obesity), a lower ICER indicates the same unit of outcome can be achieved at a lower cost (Institute of Health Economics 2012). Incremental cost–utility ratio (ICUR) involves incorporating QALY into the cost-effectiveness calculation. The Canadian Agency for Drugs and Technologies in Health (CADTH) determined that all primary bariatric procedures, compared to nonsurgical treatment over a life span, corresponded with an ICUR ranging from $6,500–$12,000 per QALY (Klarenbach et al. 2010).
Bariatric surgery has been determined to be cost-effective on a global level. A study from the United Kingdom found that the ICUR for LRYGB and LAGB, compared to standard care, was £1,500 and £1,900, respectively. The ICER over 20 years was £3,500–£12,800 for LRYGB and LAGB; however, over 2 years LAGB had an ICER of £60,800 (Klarenbach et al. 2010). A study in Portugal observed an increase of 1.9 QALY compared to medical intervention and a savings of €13,000 per patient (Faria and Preto 2013).
In the United States, an ICUR of $5,400 USD–$16,000 USD for women, and $10,700 USD–$35,600 USD for men, was calculated after gastric bypass (Klarenbach et al. 2010), and an ICER over a lifetime of $6,600 USD and $6,200 USD per QALY gained, for LRYGB and LAGB, respectively (Wang and Furnback 2013). Another American study determined that the ICUR after 10 years, would be $21,600–$38,000 per QALY, or $9,400–$12,000 per QALY over a lifetime, for LRYGB and LAGB (Klarenbach et al. 2010). The United States remains one of the most expensive healthcare systems in the world, yet the cost-effectiveness of bariatric surgery, compared to standard care, is equivalent across countries.
The bypass dominates as the most cost-effective weight loss option for obese type II diabetics. Hypertension and diabetes are by far the more expensive and prevalent comorbidities, together totaling an annual cost of nearly $2,300 USD per patient in pharmaceuticals. Cost savings after bariatric surgery account for a reduction in two-thirds, of medical expenses associated with obesity (Maggard et al. 2005).
2.3 Recurrence Rate
Weight regain occurs in 10–20 % of patients after approximately 36 months post-bariatric surgery (Sheppard et al. 2013). Different philosophies exist, as to whether weight recidivism is due to a lack of behavioral lifestyle change or simply a mechanical failure of the procedure (de Gara and Karmali 2014). Several methods exist for managing such patients. These include revisional surgery, endoscopic interventions, and medical management. The frequency of undergoing revisional surgery ranges from 2.5 to 18.4 % (Sheppard et al. 2013). Inevitably, these surgeries have higher complication rates than primary surgery (Worni et al. 2013). As such, revisional surgery due to weight regain comprises a long-term direct cost to the healthcare system that has yet to be quantified.
2.4 Causes of Revision Surgery
There are several major causal factors for patients to seek or require revisional surgery. Weight regain is one of the more common long-term reasons for requesting revisional surgery.
2.4.1 Weight Recidivism
Weight recidivism has become a major concern after bariatric surgery. Long-term studies show that over time, patients slowly regain weight, and upwards of nearly 15 % will fail to lose an excess weight loss of 50 % or more, after 5 years (Magro et al. 2008). In fact 20 % of patients will incur weight regain or insufficient weight loss. This subset of patients will gain back on average 22 kg of weight and after 36 months require revisional surgery (Sheppard et al. 2013).
2.4.2 Management Type
Management of this group of patients is complex, and considerable variance of opinion exists as to best practice. Schools of thought range from a highly mechanistic management strategy through to a solely nonsurgical approach. Mechanical/technical problems may be anastomotic/stomal pouch dilatation, fistulae, ulceration, reflux and dysphagia, or lack of restriction. Multiple solutions for these have been advocated. However, given that a multidisciplinary team is beneficial in the success of primary bariatric surgery, some proponents feel it also plays a role in the success of these revisional procedures.
It has been argued that only in a multidisciplinary environment can many of these complex issues be effectively addressed. For example, failure to address important lifestyle, behavioral and psychosocial issues, almost guarantees continued or repeat failure (de Gara and Karmali 2014). In addition, long-term dietary follow-up, outside a specialty clinic, can be costly in either a public or private healthcare system, and may be a contributing factor, to those unable to afford or have these services insured. A unifying factor that draws these issues together is appropriate patient selection. The need for appropriate follow-up, with the multidisciplinary team, is critical to ensure that patients are equipped with the tools, necessary to cope and control their weight when stresses, dietary needs, or socioeconomic situations arise. Many bariatric surgeons tend to focus solely on procedural approaches; for example, the importance of original bougie size or pouch dimensions, while failing to address the behaviors that led to sleeve or pouch dilatation.
2.4.3 Medical Tourism
A bariatric medical tourist is an individual intentionally seeking bariatric surgery outside of the province or country, and having an unsatisfactory outcome. This has become an important component of revisional surgery, and a factor in the substantial costs, associated with managing complex bariatric revision patients. Many travel to avoid the long wait times common within a public system, or personal costs, should they either have minimal or no insurance within the private healthcare system. Many patients receive negligible education on behavioral modification pre- or postop. In addition, some institutions do not follow the NIH criteria for bariatric surgery, and patients may not be psychosocially or medically optimal to succeed after surgery. Personal choice, both of procedure and institution, is an important factor.
The burgeoning LAGB failure rate has become a dominant patient group in the revision clinic. A variety of procedural failures are seen, from weight regain to band erosion or slip. While some centers (Ardestani et al. 2011) advocate for repeat laparoscopic band readjustments, so as to avoid removal, most centers find that explantation, and subsequent conversion to a definitive restrictive and/or malabsorptive procedure, is preferred (Deylgat et al. 2012). These endeavors are costly to the healthcare system.
Laparoscopic sleeve gastrectomy patients form the next important group of patients, who may require revisional surgery. Most of these are related to acute complications. Emergent complications such as leakage, bleeding, and thromboembolic episodes can represent a huge range of costly bariatric failure (Sheppard et al. 2014a, b). Later consequences of primary surgery failure, such as weight recidivism, may present demands both for the multidisciplinary team and for formal revisional surgery.
On average the revisional surgery, and care necessary to treat weight regain and complications, is 74 times more expensive than treatment of complications performed in the appropriate healthcare system ($450 vs. $37,000) (Sheppard et al. 2014a, b). It can be expected that as the number of obese individuals increase, so will the number of bariatric medical tourists, along with other inadequately selected or followed bariatric candidates, and thus the number of patients with weight recidivism.
2.5 Management Options
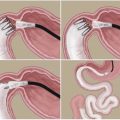
There are several options for revising patients due to weight regain. The proportion of these revisional procedures within a Canadian clinic is outlined in Fig. 2.1.
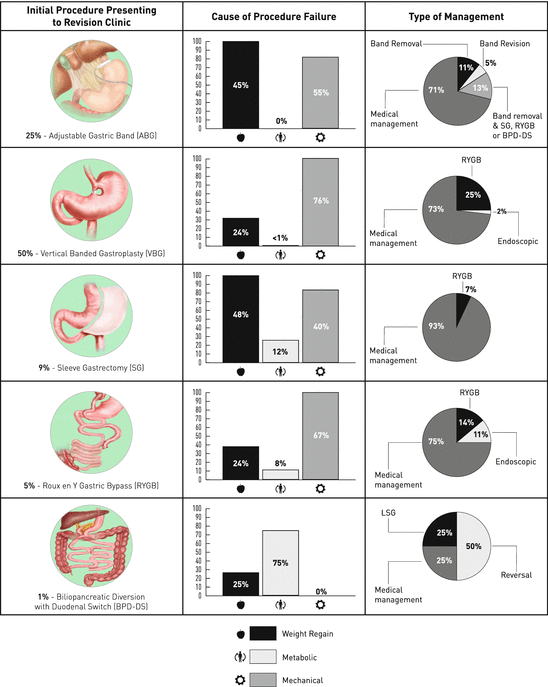

Fig. 2.1
Canadian Bariatric Revision clinic characteristics of failed primary bariatric surgery and revision surgery rates (Illustrations of bariatric procedures provided by the Centre for the Advancement of Minimally Invasive Surgery. Figure created by Maxwell Hurd, University of Alberta)
2.5.1 Revision Surgery
RYGB surgery achieves its maximal weight loss at approximately 1 year (Whitlock et al. 2013). The majority of patients then enter a maintenance phase where their weight is relatively stable. However, an average of 21 % of patients begin to regain weight at this point (Sheppard et al. 2013). The RYGB can be modified to a distal gastric bypass with revision surgery. This involves lengthening the Roux limb and effectively bypassing more small intestine. Rawlings et al. published retrospective evidence that this revision surgery is able to achieve improved weight loss (Rawlins et al. 2011). Unfortunately, there is still a paucity of evidence supporting the effectiveness of this revision strategy. For RYGB patients, converting to a duodenal switch procedure has been advocated. This is a technically challenging endeavor (Keshishian et al. 2004).
LAGB is unlike other bariatric operations, in that it does not alter the native anatomy of the gastrointestinal system. Consequently, there are multiple revision options available. LAGB can be converted to a LSG, RYGB, or a duodenal switch. Essentially the band is removed, and the subsequent operation is identical to a primary bariatric surgery. LSG has been shown to achieve significant weight loss in patients with a prior LAGB (Berende et al. 2012). However, there were 8.6 % staple-line leaks and bleeds with the LSG. This resulted in increased costs, due to reinvestigation and reoperation. This emphasizes the importance of complication rates, when considering the economic impact of a revision surgery.
Revisional surgery is inevitably more costly and complication prone than primary procedures. A recent systematic review summarized the studies of LAGB, revised to either RYGB or LSG (Coblijn et al. 2013). LSG was found to have a 5.6 % leak rate after conversion from LAGB. RYGB had a leak rate of 0.9 %. This would imply that converting LAGB to LSG is overall more costly. However, there was a wide variation in overall complication rates, for conversion to RYGB among the studies; ranging from 3.0 to 29.3 %. Ranges of this order of magnitude make it difficult for interpretation.
There is some evidence for converting LAGB to a duodenal switch procedure. A retrospective study by Topart et al. reported on 21 patients who underwent duodenal switch after LAGB surgery. However, the duodenal switch resulted in significantly more staple line leaks and bleeds, relative to the RYGB (Topart et al. 2007). Consequently, to save on the costs of reinvestigations and reoperations, LAGB is not commonly converted to the duodenal switch.
Revision surgery for a primary LSG involves conversion to either RYGB or BPD/DS. In fact LSG was originally used in a staged approach, to the RYGB and the BPD/DS, in complicated patients (Brethauer et al. 2009). LSG is now commonly done as a stand-alone bariatric procedure. There is evidence that revision to a RYGB is as effective and safe as a primary RYGB surgery (Morales et al. 2010). However, the LSG patients will incur costs needed to undergo the RYGB or the BPD/DS.
Re-sleeve, or performing a second LSG, has been described in the literature. The idea of this revision procedure is to further decrease the size of the stomach. An initial case study described this surgical approach in 2003, when a patient with a BPD/DS underwent an additional LSG (Gagner and Rogula 2003). More recently, a feasibility study reported no complications, with the re-sleeve operation for 13 patients (Iannelli et al. 2011). Unfortunately there is limited evidence for the re-sleeve procedure. Yet the possibility of a surgical procedure with less complications would result in a more cost-effective approach to revising LSG patients.
Vertical banded gastroplasty (VBG) is not commonly performed, but patients who had this procedure are now presenting for revision surgery. In fact, a recent study reports the revision rate to be 21 % (Marsk et al. 2009). VBG can be converted into a RYGB (Gonzalez et al. 2005). There is some evidence that conversion to a RYGB is a better option than simply revising the VBG (Marsk et al. 2009). Unfortunately, there were 4.8 % leaks and 1.9 % bleeds, within the first month after revision RYGB (Gagne et al. 2011). This would make RYGB a less favorable option, if the alternatives were not significantly more costly. In contrast, revision of VBG to LSG has resulted in leak rates as high as 14 % (Berende et al. 2012). Additionally, revision of VBG to BPD/DS has reported leak rates of 22 % (Greenbaum et al. 2011). There is suggestion that VBG conversion to either LSG or BPD/DS can be performed safely and will achieve further weight loss (Jain-Spangler et al. 2013). However, the evidence is primarily based on case series, and revision to RYGB is the more accepted approach.
The cost-effectiveness of revision surgery has yet to be determined. While certain economic studies have included revisional surgery for complications (Klarenbach et al. 2010), no long-term studies to date have assessed the impact of revisional surgery on the healthcare system.
2.5.2 Endoscopic Revision
Novel and innovative endoscopic strategies are advocated for primary bariatric surgery failures (Schweitzer 2004). However, the costs of these interventions have not been well documented. Endoscopic revision of RYGB procedure is becoming more established. An endoscopic transoral reduction method was recently investigated in the literature (Thompson et al. 2013). The participants had undergone RYGB surgery and were deemed to have inadequate weight loss. It is known that a larger percentage of patients with weight regain have dilated gastrojejunal junction diameter (Heneghan et al. 2012). The endoscopic approach used a suturing system to decrease the diameter of the GJ junction to 5–8 mm (Thompson et al. 2013). Experimental subjects lost a statistically significant average of 3.5 % of their preoperative weight, compared to 0.4 % in the sham-treated controls. Importantly, none of the 50 experimental patients were reported to have serious adverse events that would require future workup and gastrointestinal intervention. This is one argument for the endoscopic approach: less risk of adverse events, because of the less invasive method.
The incisionless operating platform™ (IOP) is designed to place placating sutures, within the gastric pouch. A “TransPort” device, with four channels, allows stability of the endoscopic instruments. A full-thickness fold is created and fastened with anchors connected with a suture. The overall goal of the IOP is to reduce the size of the stoma and pouch, after they are found to be dilated. This anchor system was used in a larger prospective trial, with encouraging results in the revision of 116 RYGB patients (Horgan et al. 2010). There were no significant complications associated with the procedure, and the authors reported an 18 % excess weight loss at 6 months post-IOP. Additionally, the authors provided endoscopic evidence of the anchor durability at 12 months post-procedure. Consequently, this endoscopic revision method may have better long-term weight loss.
Another device called StomaphyX™ is designed for the revision of the gastric pouch after failure of RYGB. During endoscopy the device uses polypropylene H-fasteners to create a gastric fold. After repeated folds are created in a circumferential pattern, the pouch size is reduced. A recent retrospective review by Goyal et al., reported on 53 patients who were undergoing StomaphyX™ after RYGB surgery (Goyal et al. 2013). There were no reported complications, and at 2–4 years the excess body weight loss was 4.3 %. The StomaphyX™ has also been used for revision of VBG patients. A retrospective study of 14 VBG patients undergoing revision found an average BMI decrease of 3.6 kg/m2 1 year post-StomaphyX™ (Manouchehri et al. 2011). There were no major complications with the procedure. Based on the limited evidence available, StomaphyX™ appeared to be a safe revision procedure with reasonable short-term weight loss. However, recent evidence suggests that StomaphyX™ may have poor weight loss outcome and increased morbidity compared to other available options (Eid et al. 2014).
Another method is called the over the scope clip (OTSC)™ (Ovesco, Tubingen, Germany). This method uses a Nitinol clip that is applied by an endoscope, in order to reduce the diameter of the gastrojejunal outlet. The idea is that this operation is best performed in patients with dilated GJ junctions, as identified by gastroscopy. In a recent study, 94 patients who initially had a transected vertical gastric bypass presented for treatment with the OTSC endoscopic method (Heylen et al. 2011). After OTSC, 2.1 % of the patients had persistent dysphagia, but there were no major complications. At 12 months post-OTSC, the average BMI had dropped 5.4 kg/m2.
Sclerotherapy has also been described in the treatment of weight recidivism in RYGB patients. This method involves injecting a sclerosant into the dilated gastrojejunal stomal tissue. The sclerosant elicits an inflammatory response and edema, which restricts the stomal diameter (Abu Dayyeh et al. 2012). A recent retrospective study reported 231 patients who underwent sclerotherapy after RYGB. They reported that 76 % of their cohort stabilized their weight. They also reported an average of 4.4 % of total body weight loss. However, many of their patients required more than one sclerotherapy session. As well, complications included 1 % ulceration and 2.4 % bleeds, with 1.4 % requiring endoscopic clips.
A paucity of data exists on the costs of these procedures. Dakin et al. are the first to describe the costs of endoscopic revision. IOP and Stomaphyx are said to cost equivalent to an adjustable gastric band ($18,000 USD 2012), an OTSC clip to an endoscopic retrograde cholangiopancreatography ($2,600 USD 2012), and sclerotherapy to a colonoscopy ($1,200 USD 2012) (Dakin et al. 2013). However, no literature exists on the short- or long-term cost-effectiveness of these endoscopic procedures.
2.5.3 Medical Management
For bariatric surgery to be truly effective, long-term medical, dietary, and psychosocial interventions are necessary. Weight regain after bariatric surgery is equally multifaceted (Sheppard et al. 2013).
Adherence to postoperative follow-up is important for weight outcomes in bariatric surgery patients. Weight regain is more prevalent for patients who do not receive postoperative nutritional follow-up (Magro et al. 2008; Warde-Kamar et al. 2004). At these visits, proper eating behavior and practice of physical exercise are evaluated and reinforced (Bond et al. 2004). However, failure of diet and exercise programs is well known, and the costs are almost impossible to assess.
Pharmacologic options are available for weight loss and potentially for weight regain. The medications available have been shown to achieve modest weight loss, in comparison to bariatric surgery (Yanovski and Yanovski 2014). One of the most studied is Orlistat, which is designed to inhibit lipase and prevent the absorption of fats from a meal (Heck et al. 2000). A recent meta-analysis reported that Orlistat achieves 5–10 kg of weight loss, when combined with behavioral intervention (Leblanc et al. 2011). Importantly, the weight loss was maintained for up to 24 months.
Another commonly used agent is Lorcaserin. This medication is designed as a selective agonist of the serotonin 2C receptor (Smith et al. 2009). The idea is that it reduces appetite, which subsequently reduces weight. The efficacy of Lorcaserin is similar to Orlistat, in terms of weight loss. A large randomized trial of 3,182 obese patients compared Lorcaserin to placebo (Smith et al. 2010). After 1 year, half of the Lorcaserin-treated patients achieved 5 % weight loss or more.
Solely a medical management program is not a cost-effective method for long-term weight loss. No significant difference exists in the QALY, between primary care physician follow-up and lifestyle behavior modification programs. Short-term ICER is $115,397 USD per QALY, compared to a willing-to-pay cost of $50,000 USD per QALY. Lifestyle counseling programs were only cost-effective, if the payee were to invest $400 USD per kg-year, for a loss of 10.87 kg-year (Tsai et al. 2013). Furthermore, the cost of Orlistat is €66 or $138 USD per month, resulting in an ICER of €17,000 per QALY (Lacey et al. 2005). Both Orlistat and Locaserin are not cost-effective therapies for weight loss, and only 10 % of simulations were cost-effective at $100,000 USD per QALY. To date, targeted medical interventions have not been successful and far outweigh the cost of primary bariatric surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree